|
History > 2006 > USA > Health (V-VI)
F.D.A. Tentatively Declares Food
From
Cloned Animals to Be Safe
December 29, 2006
The New York Times
By ANDREW POLLACK
and ANDREW MARTIN
After years of delay, the Food and Drug
Administration tentatively concluded yesterday that milk and meat from some
cloned farm animals are safe to eat.
That finding could make the United States the
first country to allow products from cloned livestock to be sold in grocery
stores.
Even if the agency’s assessment is formally approved next year, consumers will
not see many steaks or pork chops from cloned animals because the technology is
still too expensive to be used widely.
But the F.D.A.’s draft policy touched off an immediate storm of criticism from
consumer groups, as well as some concerns from meat and dairy companies worried
about consumer reaction.
“At the end of the day, F.D.A. is looking out for a few cloning companies and
not for consumers or the dairy industry,” said Joseph Mendelson, legal director
for the Center for Food Safety, an advocacy group.
Mr. Mendelson and other consumer representatives argue that the science backing
the F.D.A.’s decision is shaky and that consumer surveys show that most people
are opposed to cloning animals, let alone eating them. Some also said that
cloning causes harm to the animals involved and could pave the way for human
cloning.
Opponents hope to bring Congressional pressure to bear to derail the policy
before it becomes final or at least to require that such foods be labeled so
consumers can choose to avoid them. F.D.A. officials said that it was unlikely
that labeling would be required because food from cloned animals is
indistinguishable from other food, although a final decision about labeling has
not been made.
Senator Patrick J. Leahy, Democrat of Vermont, yesterday called for a “careful,
deliberative and open process” before cloned animals are approved for food.
The F.D.A.’s finding comes more than six years after the agency first decided to
study the matter, after recognizing that the advent of cloned farm animals
raised a food safety issue. After that study, the agency in 2003 gave a
tentative approval to cloned animals for food. But the F.D.A. retreated after
its own advisory panel found there was insufficient scientific backing for that
conclusion.
This time, F.D.A. officials said they had substantial new data, which they
presented yesterday in a nearly 700-page “draft risk assessment.”
The officials denied the contention from some critics that the policy was
announced during a holiday week in order to reduce publicity, saying it had
taken until now to analyze the data and obtain comment from other government
agencies.
The assessment concluded that milk and meat from cloned cows, pigs and goats,
and from their offspring, were “as safe to eat as the food we eat every day,”
Stephen F. Sundlof, the F.D.A.’s chief of veterinary medicine, said in a
telephone call with reporters.
Mr. Sundlof said that by law the agency could consider only the scientific
issues, not consumer demand or the ethics of cloning.
While animal cloning has always been legal, since 2001 there has been a
voluntary moratorium on the sales of milk or meat from such animals to give the
F.D.A. time to study the matter. Some experts say that some products from clones
or their offspring have probably nonetheless made their way into the food
supply.
The moratorium will stay in place until the new policy is completed, after a
90-day period for public comment and additional time for the F.D.A. to review
the comments. Mr. Sundlof said he could not say when the final policy would be
ready, though it might be by the end of 2007.
Even then, the moratorium would remain for products from sheep, the F.D.A. said,
because there was not enough evidence of their safety. No one has yet succeeded
in cloning chickens or other poultry.
The finding was hailed by cloning companies, which have been struggling to build
a business. It also drew praise from some farmers and breeders who have already
made clones of their prized livestock but have had to pour milk down the drain
and keep their meat off the market.
They say that cloning is just another breeding technique, like artificial
insemination or in-vitro fertilization.
“This just sort of lifts the stigma of the clones,” said Bob Schauf, a Holstein
breeder and dairy farmer in Barron, Wis., who had two of his prized cows cloned.
He said his family and the families of his employees have been drinking the milk
from those clones rather than see it go to waste. But dairy marketers have
expressed concern.
A survey conducted last summer by the International Dairy Foods Association, an
industry trade group, found that 14 percent of women would turn away from all
dairy products if milk from clones were introduced into the food supply. The
association surveyed women because its research has found them to be the main
household decision makers on dairy products.
The American Meat Institute, while saying yesterday that cloning was safe, also
urged the F.D.A. to be cautious about approval “if most consumers are unwilling
to accept the technology.”
A poll this month from the nonprofit Pew Initiative on Food and Biotechnology
found that while most consumers knew little about animal cloning, 64 percent
said they were uncomfortable with it, with 46 percent saying they were “strongly
uncomfortable.”
F.D.A. officials said no other country had yet approved food from cloned
livestock, although some are considering it. That raised the prospect that
American exports of milk or meat could be blocked by certain countries if they
contain products from cloned animals. An official in the Washington delegation
of the European Union said politicians and consumers in Europe would no doubt
debate the issue.
Carol Tucker Foreman, director for food policy at the Consumer Federation of
America, said consumer groups would ask food companies, retailers and restaurant
chains to shun products from cloned livestock.
That raises the possibility that some food companies will label their products
“clone free,” just as some now label milk as not coming from cows injected with
growth hormone.
Cloning involves putting an animal’s DNA into an egg thats own DNA has been
removed. The resulting embryo, after being implanted into a surrogate mother,
makes a genetically identical copy of the original animal.
But even if two animals have identical genes, they can turn out differently if
those genes are turned on or off at different times. And studies have shown that
patterns of gene activity are different in embryos created by cloning compared
with embryos created by the fusing of sperm and egg.
These differences are presumed to account in large measure for the low success
rate of cloning. Fetuses can grow unusually large, posing a risk to the
surrogate mother. Many clones die during gestation or shortly after birth. Some
are born with deformed heads or limbs or problems with their hearts, lungs or
other organs.
But the F.D.A. said that obviously sick and deformed animals were already barred
from the food supply. It added that clones that survived past the first few days
“appear to grow and develop normally” and that healthy adult clones were
“virtually indistinguishable” from noncloned livestock, making their meat or
milk safe.
The draft assessment based its conclusions on an extensive review of scientific
literature on cloning as well as on studies, some done by cloning companies,
comparing the composition of the milk, meat and blood of cloned animals and
conventional animals.
Mr. Sundlof said the agency also found that cloning “poses no unique risks to
the health of animals” beyond those seen with other forms of assisted
reproduction such as in-vitro fertilization. The frequency of problems is higher
with cloning, however, perhaps because it is a newer technology. The first
cloned mammal, Dolly the sheep, was born in 1996.
The F.D.A.’s announcement, by paving the way for the end of the moratorium,
could make it easier to persuade farmers and breeders to pay $15,000 to copy a
prized bull or dairy cow.
“I think that this draft is going to provide the industry the comfort it needs,”
said Mark Walton, president of ViaGen, a cloning company based in Austin, Tex.,
that has yet to turn a profit after five years.
Industry officials estimate there are now only about 500 or 600 cloned cows in
the United States, out of tens of millions of beef and dairy cows. There are
roughly 200 cloned pigs.
Experts say that cloning is too expensive to be used to make animals only to
then grind them into hamburger or even to milk them. Rather, farmers and
breeders are cloning prized livestock so they can then be used for breeding
using more conventional means of reproduction.
That means that most food from cloning would come from the sexually produced
offspring of the cloned animals. The F.D.A. said milk and meat from such
offspring were safe, because any abnormalities in clones do not carry into the
next generation.
The agency’s assessment did not include genetically modified animals, in which a
foreign gene is introduced. The agency is still deciding whether to allow the
first of those, a fast-growing fish, into the food supply.
F.D.A. Tentatively Declares Food From Cloned Animals to Be Safe, NYT,
29.12.2006,
http://www.nytimes.com/2006/12/29/business/29fda.html
Dialysis in N.Y. Lags
as Diabetes Ruins
Kidneys
December 28, 2006
The New York Times
By RICHARD PÉREZ-PEÑA
Tens of thousands of people across the
country, their kidneys ruined by Type 2 diabetes, have been forced into the grim
routine of dialysis care, and in New York, those patients routinely receive some
of the worst treatment, government records show.
At New York dialysis centers, those being treated are more likely to suffer from
anemia and are less likely to have enough impurities and excess fluid removed
from their blood, allowing more damage to their bodies, according to the
records.
Experts say the disparity is caused in part by the fact that New York is
dominated by small dialysis providers, many of them run by people with little
background in medicine who entered the business to meet the surging demand.
Many of the smaller centers provide good care, experts say, but a lot also lack
the money and staff training to compete on a quality-of-care basis with the
national dialysis chains that dominate the market across the rest of the
country.
Newly released patient data show that people who receive their dialysis from a
national chain generally fare better than those treated by an independent
provider.
But the chains are largely blocked from operating in New York by a state law
that effectively bars publicly traded companies from owning health care
facilities in the state.
“With the need for dialysis on the rise, the department is questioning whether
it makes sense not to allow these large corporations to participate,” said
Jeffrey W. Hammond, a Department of Health spokesman.
In 1980, fewer than 50,000 people in the United States needed dialysis to do the
work of their kidneys; today, there are more than 350,000, including roughly
24,000 in New York. In 1980, diabetes was the primary cause of kidney failure
for fewer than 6,000 dialysis patients; today, the figure is about 150,000.
Survival for them is an ordeal, at best.
At a typical dialysis center, patients come in three times a week, typically for
four hours at a time. They sit in rows of recliners, dozing, watching television
— anything to take their minds off the machines, needles and tubes that siphon
blood from their bodies, clean it of impurities like urea, and pump it back in.
It is surprisingly quiet; patients are so beset by side effects like fatigue,
cramps or thirst, that mere conversation seems like an effort.
For all but a few, holding a job is out of the question. Most will never be
healthy enough to qualify for a transplant that would free them of this burden,
and there are far too few donated kidneys, anyway.
New drugs and dialysis techniques have improved their chances of survival since
the 1980s, despite the fact that patients today are older, heavier and sicker.
Even so, the average dialysis patient spends 15 days a year hospitalized, and
the death rate is about one in five each year.
“I want to say it’s a rough life, but it hardly is a life,” said Denise Bembury,
a dialysis patient who lives in Brooklyn. “I wouldn’t put this on anybody.”
Across the country, five companies own or operate almost two-thirds of the 4,800
dialysis units. Two of them, Fresenius Medical Care and DaVita, have more than
half the market. But in New York, the big five run about 20 percent of the
roughly 250 centers, by far their lowest share in any state.
The State Department of Health has helped the national chains work around the
law that bars publicly traded companies from owning health facilities. This has
allowed them to open some centers here, but the companies say the approval
process remains long and difficult, dampening their interest.
The ownership restriction, in place for at least 50 years, state officials said,
was enacted when there was no such thing as a dialysis center, and was intended
to ensure that hospitals are responsive to local concerns, not to far-flung
shareholders. Though the Health Department talks of changing the law, no one has
made it a priority, and not even the large chains have pushed the issue in
Albany.
Recent reports on the quality of dialysis care by the federal Centers for
Medicare and Medicaid illustrate New York’s cause for concern. The centers
examined a sampling of Medicare patients’ dialysis records in each of 18
regions, one of them New York State.
Medicare pays for almost 90 percent of dialysis in this country, and in the
reports, for 2003 and 2004, New York ranked worst in all three of the most
commonly used quality measures. Those measures are how likely patients are to
have enough excess fluid like water removed from their blood during dialysis,
how likely they are to have enough impurities like urea removed, and how likely
they are to be anemic or severely anemic because of the treatment.
Those scores have improved in New York over the last decade, but not as quickly
as they have nationwide, and New York’s numbers were actually worse in 2004 than
in 2003.
The federal agency does limited comparisons of individual dialysis centers,
which show that nationally, 4 percent of them have unusually poor patient
survival rates, defined as at least 20 percent below average. In New York, 12
percent do. Federal officials caution against putting much stock in any one
center’s numbers, but they say the regional picture clearly shows a problem.
The federal data do not draw any conclusions about the cause of the disparity.
But experts said they believed the quality of care was affected by the high
number of smaller, less experienced providers in the New York market. In fact,
in New York City, one-fifth of the centers operating earlier this year had
existed for less than five years.
A recent report by the United States Renal Data System Coordinating Center, a
quasi-governmental agency that compiles the records of most dialysis patients,
shows that patients at the major chains were less likely to die than those
treated by smaller companies.
According to the center, the numbers were adjusted to account for differences
that might affect patients’ risk, like age and sex, whether they had an
underlying disease like diabetes and how long they had been on dialysis. The
independents were defined as not being part of a major chain or a hospital.
Four of the five largest chains had adjusted death rates 7 percent to 10 percent
lower than the independents had as a group in 2004, and one chain had higher
rates, according to the center’s most recent annual report. Since then, two
major chains, including the one with the highest death rate, have left the
business, and two new ones have been formed.
Patients at the small companies were much more prone to infections that led to
hospitalization or death. The small companies also lagged in nondialysis care
that dialysis centers usually take over, like ensuring that patients get
vaccinations for influenza, pneumonia and hepatitis B. Their diabetic patients
were less likely to have tests that monitor blood sugar control.
Some independent operators say that large companies can “cherry pick” healthier
clients. But the government’s figures show that the national chains’ patients
are actually sicker when they begin dialysis — more anemic, more overweight, and
with more advanced kidney disease.
Researchers and state and federal officials say they have known, or at least
suspected, for years that patients fare better at the major chains, but they
cannot be sure why.
One factor believed to play a role is that the average Medicare reimbursement
rate for a dialysis session, $140 to $150, has changed little since the 1970s,
making it difficult for the smaller operators who cannot realize economies of
scale. Tight operating margins have left fewer small providers nationwide,
although their numbers have grown in New York, where the chains have not been
able to operate freely.
Dr. Allan J. Collins, director of the United States Renal Data System
Coordinating Center, said: “We do know that the large organizations have an
enormous advantage in resources because they can demand discounts from suppliers
on drugs and equipment, and that can translate to better staffing, better
training. The chains also tend to be more systematic and standardized in their
procedures.”
Michael Paget, executive director of the National Renal Administrators
Association, which represents large and small dialysis companies, said he was
not familiar with the Coordinating Center’s research that indicates one group
performs better than the other. The center first included those comparisons in a
report last year, and first included the mortality and hospitalization
breakdowns this year.
“I don’t think you can generalize about the independents, and there are many
that do an excellent job,” outperforming even the best of the major chains, he
said. But he acknowledged that bigger companies probably had an edge in
training.
The federal government and most states, including New York, do not set training
or educational standards for dialysis technicians, the workhorses of a dialysis
center. (Federal rules require only that the centers have full-time or part-time
doctors, social workers and nurses on staff.)
National chains customarily provide technicians and other staff members with
months of initial training and occasional re-training, instruction that can
boost the quality of care and that independent companies, with their tighter
operating margins, have a hard time affording, experts say.
Ms. Bembury, 44, uses an independent center, Nephrocare, a three-year-old unit
on Atlantic Avenue, in the Weeksville section of Brooklyn. She goes there, she
said, because her doctors referred her there. Like many patients, she has no
idea how it compares with other centers, or how to find out.
Until earlier this year, she was a social worker and an avid cook. Now, she is
on disability, and her companion of more than 30 years prepares meals. They have
six children, and she wonders how the four youngest, all teenagers, will manage.
“I’m thirsty all the time, and tired,” she said.
Nephrocare was one of a few dozen centers in New York with an abnormally high
death rate in 2004, though not in 2005, and in both years, it had an unusually
high number of patients with uncontrolled anemia, Medicare records show. An
administrator at Nephrocare said that none of the owners or staff would be
interviewed, and several other large, independent centers around the city gave
the same response.
There are no rules as to who can own or manage a dialysis center. Doctors
control some. Others are run by people with no background in health care. State
officials say they conduct a “character and competence” review, and look at
owners’ finances.
Under Medicare rules, states must inspect most centers every three years, though
troubled centers are visited more often. Even when there are persistent
problems, regulators would rather coax a center into improving than shut it,
even temporarily, because the dialysis supply barely keeps up with demand.
State officials said they could recall only one center being forced to close
this decade, and Medicare gave that order, not the state. The experience was
painful, they said, as patients had trouble finding other centers nearby with
spaces, or stations, available.
“We had 403 people who had to find other stations in New York City,” said Mr.
Hammond, the department spokesman, “and that is not something we encourage or
want to happen.”
Dialysis in N.Y. Lags as Diabetes Ruins Kidneys, NYT, 28.12.2006,
http://www.nytimes.com/2006/12/28/nyregion/28dialysis.html
The Doctor’s World
So Many Advances in Medicine,
So Many Yet
to Come
December 26, 2006
The New York Times
By LAWRENCE K. ALTMAN, M.D.
After President Dwight D. Eisenhower suffered
a heart attack in the middle of the night on Sept. 24, 1955, his physician told
Mamie Eisenhower to snuggle with her husband in bed to keep him warm.
The physician, Dr. Howard M. Snyder, injected morphine and other drugs, none
specific for a heart attack or for Eisenhower’s falling blood pressure and
irregular pulse. Dr. Snyder, a general surgeon, let Eisenhower sleep until noon
at Mamie’s family home in Denver, where he was staying. Then he called a
cardiologist to do an electrocardiogram. Later, the president went by car to a
hospital. There, he was largely confined for almost seven weeks to bed, chair
rest and limited physical activity.
Eisenhower’s care shows how little doctors could do for heart attacks in 1955,
three years before I entered medical school, where we were taught that medicine
would be a lifetime of learning. My professors could not have known how powerful
the advances that followed would be.
As a reporter for The New York Times for 37 years, I have witnessed many
important medical events, from new treatments to new diseases. In reflecting on
that panorama, it is clear that technology has accounted for the greatest
changes in medicine. Technology has improved laboratory testing; allowed for the
development of CT scans, magnetic resonance imaging exams and positron emission
tomography, or PET, imaging to improve diagnostic accuracy; and produced new
drugs and devices. Basic science, too, has deepened our understanding of
disease, and much of that work depends on technology.
At the same time, the care for many ailments has been greatly improved by
ancillary developments like better nursing care, newer antibiotics, transfusions
of platelets to prevent bleeding, the insertion of monitoring tubes in major
veins, and better organization of some services.
The gains, though, have also led to new challenges, like the risk of developing
Alzheimer’s disease, strokes, arthritis and other ailments that increase in
frequency with age.
When Eisenhower had his heart attack, heart disease was the No. 1 killer of
Americans, and it remains so today. But more deaths would occur without these
advances. Men and women with chest pain are now told to call 911, setting off an
emergency response that often results in medical care within minutes, not the
hours as in Eisenhower’s case.Emergency medical technicians sent to the scene
can transmit electrocardiograms to a base hospital. They can inject drugs to
dissolve clots and can correct potentially fatal heart rhythms at the scene or
in an ambulance on the way to a hospital. These measures can stop a heart attack
before it scars heart muscle.
Coronary-care units for round-the-clock monitoring were just being created
during my training. Now they are standard. Doctors urge patients to become
physically active as soon as possible, and most go home a few days after a heart
attack.
Eating sensibly and exercising regularly are today’s central messages for
preventing heart disease. A variety of drugs are widely prescribed to lower
cholesterol and high blood pressure, two problems that increase the risk of
heart attacks.
Angiogram X-rays can detect coronary arteries that are clogged with deposits of
cholesterol and other fats. An angioplasty procedure can clear the arteries, and
stent devices can keep the new channels open. Heart bypass surgery is available
when angioplasty and stents will not work.
For me, an electrifying moment came in the early 1960s. Dr. Bernard Lown of
Harvard reported how a shock of direct current applied to the skin over the
chest at a precise point in the heart beat could safely correct a number of
dangerous heart rhythms. Alternating current could not be used because such
shocks were often lethal. But application of the direct current technique helped
make open-heart surgery — and many other advances — possible.
In time, pacemakers came along. Now we have implanted devices that serve as both
pacemakers and defibrillators. They can automatically detect a life-threatening
abnormal rhythm and then shock the heart.
Vice President Dick Cheney, who had a sophisticated pacemaker and defibrillator
implanted in 2001, is perhaps the most prominent recipient of these advances.
Few people appreciate that medicine has advanced more since World War II than in
all of earlier history. Newer drugs and devices and better understanding of
disease mechanisms have vastly improved the care of patients. For male babies
born in this country in 1960, the life expectancy was 66.6 years; for female
babies, it was 73.1 years. In 2004, the figures, respectively, were 75.2 and
80.4. Medical advances account for much, though not all, of the gain.
As a specialist in internal medicine in the 1960s, I could prescribe about 7,000
drugs approved by the Food and Drug Administration. That number is now more than
11,700 drugs, including many new classes of medication.
Powerful drugs help patients live longer with heart failure.
The mid-1960s saw the introduction of Lasix (furosemide), a vastly more powerful
diuretic than any before it. With its help, patients with heart disease and
other disorders, whose bodies swelled with gallons of fluid, could eliminate the
fluid through urination within hours.
Another drug, thalidomide, has gone through an amazing resurrection. In the
1960s, the F.D.A. kept thalidomide off the American market (though some doctors
gave out free samples). Its use in other countries soon showed that the drug
produced flipper-like arms and other ghastly birth defects among children born
to mothers who took the drug as a sedative or for morning sickness. Now
thalidomide is approved for a complication of leprosy, and doctors prescribe it
off-label for some cancers.
Anti-rejection drugs have allowed organ transplant surgery to flourish, though
no one has solved the organ donor shortage that deprives many people of a chance
at longer lives.
Although doctors had measured blood pressure, few believed until the 1960s that
lowering high blood pressure would prevent complications like strokes, heart
attacks, loss of vision and kidney disease. In medical school, a standard
therapy was the sedative phenobarbital. Now, tens of millions of Americans take
more effective medications — like diuretics — to prevent these hazards.
New drugs are only part of the story. A million test tube babies would not have
been born without the in vitro fertilization technique introduced in 1978. And
immunizations introduced in recent years protect children against infections
that include chicken pox, hepatitis A and B, measles, mumps and pneumococcal
pneumonia.
As an epidemiologist at what is now the Centers for Disease Control and
Prevention, I helped investigate some of the last outbreaks of polio in the
United States. We used vaccine-laced sugar cubes that were easier to administer
than injections in a public health emergency. I also helped investigate
outbreaks of botulism, a food-borne disease produced by one of the deadliest
toxins known. A few researchers mused about turning the toxin into a useful drug
for serious neurological problems. That vision became Botox. But none of us
dreamed that the most common use would be cosmetic, to temporarily erase the
visible wrinkles of aging.
As the son of a radiologist whose office was in our home, I grew up seeing
conventional X-rays displayed on my father’s light boxes. When I went to London
in 1973 to report on the first brain CT scanner, I was astonished to see how it
could detect tumors, strokes and other disorders that never could be seen on
X-rays. I recalled all the patients with neurological symptoms who had to
undergo a special X-ray procedure known as a pneumoencephalogram. In it, a
needle was inserted through the back to remove spinal fluid and to inject air to
outline structures in the brain. The technique was painful and unable to detect
the tiny lesions that are now seen on scans.
Later versions of CT, M.R.I. and PET scanners revolutionized medical practice.
These scanners have simplified the decision-making process, allowed the
development of simpler and safer procedures, and eased suffering for patients.
Imaging technology also has reduced the need for exploratory surgery to detect
various abnormalities. Such operations often required a long incision and a
lengthy recuperation. Now, CT scans can detect an abdominal abscess, for
example, and have reduced what once required three operations to one.
Better imaging has also allowed radiologists to do procedures they could not
have imagined.
For me, a vivid example of that occurred when I was training as a specialist in
internal medicine in the late 1960s. The patient needed scores of blood
transfusions because of intermittent rectal bleeding. Standard techniques failed
to detect the source of the bleeding in the bowel. An attending physician told
us to stop the transfusions and let the patient die because he was depleting the
blood supply. In desperation, we asked a radiologist to use new angiographic
techniques to pinpoint the bleeding and stop it. The effort saved the man’s
life.
Medical instruments made from fiber optics have enabled doctors to peer into
many areas of the body that previously were inaccessible or that required major
surgery.
One example, colonoscopy, detects intestinal polyps before they become
cancerous. Another involves the repair of torn ligaments and tissues in knees
and other joints.
Artificial joints are now implanted in patients whose hips and knees have been
severely damaged by arthritis and injuries. Video monitors and laparoscopic
instruments allow surgeons to remove diseased gall bladders through a few small
incisions instead of one long cut that severed abdominal muscles and led to long
and painful healing. To do the new procedures, surgeons had to develop manual
dexterity and other skills entirely different from those used in traditional
operations.
In ophthalmology, cataract patients once had to stay immobilized in a hospital
for about 10 days. Sandbags were positioned to prevent any movement of their
head to protect the newly implanted lens. Now a technique called
phacoemulsification allows a lens to be implanted in a simpler cataract
operation performed as an outpatient procedure.
Some major advances in surgery did not involve technology or instruments.
Short-acting anesthetics like fentanyl now allow many patients to go home a few
hours after an operation. In my medical school days, surgeons were learning the
critical importance of monitoring fluid and vital chemicals known as
electrolytes before and after an operation. Such regulation is now standard.
For breast cancer, radical mastectomy was virtually the only choice of surgery.
We read studies from doctors in Canada and Europe who since 1939 had reported
benefits from radiation and simpler operations for breast cancer. But our
professors, virtually all men, made disparaging remarks about the quality of the
work, and held dogmatically to a thesis that the more tissue removed, the better
the outcome.
Then consumerism in medicine grew, more women became doctors, mammography was
used more widely along with other advances to detect breast cancer earlier, and
the government invested more in research on breast cancer. From these changes,
doctors began to understand that the cancer was systemic and not confined to the
breast. The studies documented that simpler and less disfiguring procedures,
often combined with radiation and drugs, were safe treatments.
We have also learned that there are many kinds of breast cancer, lymphomas and
other tumors, subtypes determined only by newer laboratory tests. This can be
crucial to offering optimal therapy.
Ulcers are another ailment that doctors now understand in a new way. In 1984,
two Australian doctors, Barry J. Marshall and J. Robin Warren, reported
scientific evidence that the H. pylori bacterium, not stress, caused most
stomach ulcers. Many doctors dismissed the finding as nonsense, and criticized
me for reporting what they knew could not be true. Soon antibiotic treatment for
ulcers made a rarity of the common ulcer treatment: surgical removal of parts of
the stomach. In 2005, Dr. Marshall and Dr. Warren shared a Nobel Prize.
Doctors can now more effectively treat more diseases, but there are now more
diseases to treat.
During my training, most professors said that all diseases were known. That
hubris left doctors unprepared when AIDS came along in 1981 to cause one of
history’s worst pandemics. H.I.V. has infected an estimated 60 million people
and killed 25 million of them.
In 2002 and 2003, a new disease, severe acute respiratory syndrome, or SARS,
spread from China to Canada and elsewhere. I began covering SARS with the first
recognition of the disease and continued as scientists, in a rare World Health
Organization collaborative effort, quickly identified the cause: a new
coronavirus. The findings allowed health officials to act swiftly to stop the
outbreak.
One of the first articles I wrote for this newspaper, in 1970, was about Lassa
fever, a hemorrhagic viral infection discovered in Africa. The virus was
isolated from a missionary nurse who flew to New York City from Nigeria for
care. She survived. But a researcher at Yale died while trying to identify the
virus. Among other new diseases are Marburg, Ebola and Legionnaire’s. Still
others, like West Nile fever, have moved from one area of the world to another.
For decades, the West Nile virus caused outbreaks in Africa and Europe. In 1999,
West Nile appeared in the Americas, in New York City. Since then, it has spread
widely and quickly through the United States and Canada to cause encephalitis
and other problems.
As malaria and many other so-called tropical diseases disappeared from the
United States, most doctors did not understand their continued importance in the
era of jet travel, which allowed someone to become infected in one part of the
world and return home before falling ill.
Eradication of smallpox has shortened the list of diseases by one. This is
arguably medicine’s greatest triumph because it is the only naturally occurring
disease we have wiped out. As an epidemiologist working in Africa in the 1960s,
I saw lots of smallpox, and took part in the first efforts to eradicate it. In
1975, I covered its successful eradication from India.
More recent therapies have improved the outlook for many patients, but are not
cures. For example, the decline in heart attacks is offset by the rise in heart
failure, which, as it worsens, makes patients progressively short of breath.
And we still do not know, for example, what causes most ailments, or the precise
biological mechanisms of basic life events like labor. Though doctors have long
stressed the importance of prevention and public health, they and society have
been slow to take strong action. Our medical school class was lucky to have a
good course in preventive medicine because epidemiology was not widely taught
elsewhere. To me, Berton Rouche, the New Yorker writer, arguably taught doctors
more about public health than all medical schools combined through his medical
detective stories about infectious and communicable diseases.
At the time, anyone who went into preventive medicine and public health was
assumed to have graduated at the bottom of the class. A shingle on Park Avenue
was the measure of success, not saving lives in poor countries. Now students are
eager to study global health.
We may snicker over Eisenhower’s treatment. But imagine the laughter in 2056 as
people look back at the brand of medicine and public health that we consider so
sophisticated today. For all that doctors have learned in the last half-century,
we are ignorant about far more.
So
Many Advances in Medicine, So Many Yet to Come, NYT, 27.12.2006,
http://www.nytimes.com/2006/12/26/health/26docs.html
As Minds Age, What’s Next?
Brain
Calisthenics
December 27, 2006
The New York Times
By PAM BELLUCK
PROVIDENCE, R.I. — Is there hope for your
hippocampus, a new lease for your temporal lobe?
Science is not sure yet, but across the country, brain health programs are
springing up, offering the possibility of a cognitive fountain of youth.
From “brain gyms” on the Internet to “brain-healthy” foods and activities at
assisted living centers, the programs are aimed at baby boomers anxious about
entering their golden years and at their parents trying to stave off memory loss
or dementia.
“This is going to be one of the hottest topics in the next five years — it’s
going to be huge,” said Nancy Ceridwyn, co-director of special projects for the
American Society on Aging. “The challenge we have is it’s going to be a lot like
the anti-aging industry: how much science is there behind this?”
Dozens of studies are under way. Organizations like AARP are offering tips on
brain health. And the Alzheimer’s Association conducts hundreds of Maintain Your
Brain workshops, many at corporations like Apple Computer and Lockheed Martin.
At least two health insurers are pushing brain health. MetLife is giving
prospective clients a 61-page book it commissioned called “Love Your Brain.”
Humana will provide, free or deeply discounted, $495 worth of brain fitness
software to some four million older customers, and offers “brain fitness camps”
with the software at computer stores and community colleges.
There are Web sites like HappyNeuron.com, which offers subscribers cranial
calisthenics, and MyBrainTrainer.com, marketed to anyone who “ever wished you
could be a little quicker, a little sharper mentally.”
And Nintendo’s Brain Age, a video game intended for baby boomers and their
elders, features simple math, syllable-counting, word memory activities and the
quick reading aloud of passages from the likes of Poe and Dickens, which “gives
your prefrontal cortex a workout,” the instructions say.
“I just felt that, Hey, this is something I ought to do,” said Roy Gustafson,
85, who tried it at a Nintendo promotion at his Redmond, Wash., retirement
community. He quickly got top scores (his “brain age” was low 20’s), and decided
to quit while ahead. But almost daily, he plays the Sudoku games in the handheld
device, saying, “It keeps me alert.”
Whether the hopes for brain health programs are realistic is still largely
unknown, scientists say.
Certainly most brain-healthy recommendations are not considered bad for people.
They do not have the potential risks of drugs or herbal supplements. And things
like physical exercise and Omega-3 fatty acids help the body, even if they do
not end up bettering the mind.
“All of the things are good for you to do in general,” Dr. Elizabeth Edgerly, a
clinical psychologist with the Alzheimer’s Association, said. “Do I have
concerns? Yes. We’re very cautious. Is it going to mean you can remember where
you left your car keys? We can’t say that.”
Still, the appeal of the programs is strong.
Epoch Senior Living in Providence is among the many assisted living facilities
with “brain fitness centers.” Surrounded by posters of Einstein, Rodin’s
“Thinker,” and “Brain Facts” (“one billion glial cells in the human brain”),
residents spend an hour a day for eight weeks doing computer exercises involving
recalling story details and distinguishing similar-sounding syllables.
David Horvitz, 92, an Epoch resident, said, “It did improve my concentration,
particularly when I read. Before, my mind would wander and I’d have to reread
passages several times. It also seems to me that I’m remembering names a little
bit better.”
Emeritus Assisted Living, a chain, started a brain health program for residents,
their families, staff members and people in the community. So far, centers in
Florida, Massachusetts and South Carolina offer “brain-healthy” foods like
salmon and walnuts, activities like spelling bees and reminiscing games, prizes
to staff members for recalling brain health trivia, and a “brain health
self-assessment” questionnaire asking, among other things, if people play
challenging board games, walk 10,000 steps a day, or eat flax seed three times a
week.
The brain program at the Isle at Emerald Court in Tewksbury, Mass., an Emeritus
facility, includes a five-day-a-week regimen of leg lifts and stretches on the
burgundy jacquard lobby chairs, influenced Ray Decker to choose the center for
his mother, Joan, 75, who is in the early stage of Alzheimer’s.
“Those types of things may stimulate her brain and, despite her debilitating
disease, she actually may come back a little,” said Mr. Decker, 57, who plans to
adopt brain-healthy activities. “I think that this will keep my mother healthy
for some time to come, actually extend her life in a mental and physical
manner.”
While there is encouraging animal research, experts say human studies have
generally relied on observations of people with healthier brains, but have not
tested whether a particular behavior improves brain health. Perhaps people with
healthier brains are more likely to do brain-stimulating activities, not the
reverse.
“Right now,” said Dr. Marilyn Albert, director of cognitive neuroscience at
Johns Hopkins University, “we can’t say to somebody, ‘We know that if you walk a
mile every day for the next six months, your memory’s going to be better.’ We
don’t know that if you do certain kinds of puzzles it’s going to have a
benefit.”
In addition, few scientists believe brain health activities prevent dementia,
only that they might delay it.
The strongest evidence suggests that cardiovascular exercise also probably helps
the brain, by improving blood circulation, experts say.
“What’s good for your heart’s probably good for your head,” said Dr. Lynda
Anderson, chief of health care and aging studies at the federal Centers for
Disease Control and Prevention, which last year received the first Congressional
appropriation to study brain health.
Similarly, Dr. Albert said that heart-healthy foods were probably brain-healthy
foods.
As for brain-training exercises, studies show improvement from them, though not
necessarily in real-life activities, said Dr. David A. Loewenstein, professor of
psychiatry and behavioral sciences at the University of Miami medical school.
In a National Institute on Aging study, people given at least 10 hours of
training in memory, reasoning or processing speed showed improvement, which held
five years later. People reported slightly less difficulty in everyday skills,
like handling medication and making telephone calls, but most of those results
were not significant, researchers reported.
Dr. Loewenstein, meanwhile, found that people with early Alzheimer’s who were
trained in real-life tasks like face-name recognition and balancing checkbooks
improved significantly in those skills. People given computer memory and
concentration games and crossword puzzles did not do as well on real-life tasks,
although many thought they were improving, he said.
“Just because you’re able to recall a story better after six weeks may not mean
that it’s had any demonstrable effect on everyday life,” Dr. Loewenstein said.
Posit Science, a San Francisco company that makes the brain fitness software
used by Epoch and Humana, said its own studies, some published, showed that its
software improved memory and mental focus.
“We’ve seen more than 10 years of improvement,” said Jeff Zimman, the company’s
chief executive. “In processing speed, people who were on average 80 years old
were performing like 30-year-olds in speed at those tasks.”
Posit, one of several making such software, hopes to adapt it for people with
early Alzheimer’s, AIDS-related dementia and schizophrenia. Mr. Zimman envisions
other uses: corporations hoping to improve brains of older employees; sports
enthusiasts and hobbyists honing, say, bird-watching skills.
Emeritus Assisted Living has partnered with Dr. Paul Nussbaum, a
neuropsychologist advocating social, mental, spiritual, nutritional and physical
ways to promote brain health, to make its 180 homes “brain health centers for
the community,” said Chris Guay, a divisional director of operations. The Isle
at Emerald Court hands out brain-shaped stress balls and plans to fly a brain
flag out front. One administrator tried stimulating her brain by writing with
her opposite hand (with barely legible results). The maintenance director wears
a pedometer and gives them to visitors. An Emeritus center in Florida is
lobbying grocery stores for brain-healthy food displays.
Mr. Guay said he hoped the program would attract “more people to fill our
buildings” and “help us retain employees.”
Some experts say even if there is little cognitive benefit, there may be psychic
benefit to mental exercises.
“I feel my brain is better,” said Dorothy Pereshluha, 84, a resident at Isle at
Emerald Court, who had trouble finding her room and remembering names when she
moved in.
Alice Babulicz, 75, a resident at Wartburg Assisted Living in Mount Vernon,
N.Y., which uses brain fitness software, said she paid more attention in church
and was so energized that “now I can walk four or five blocks.”
And Marcia Mittleman, 88, who took Epoch’s course twice, with graduation and a
medal, said that psychologically, it “filled a void.”
Asked if her cognitive function improved, she replied, “Did it make me smarter?
No.”
Suddenly, she scanned the room. “Did anyone see my walker?”
As
Minds Age, What’s Next? Brain Calisthenics, NYT, 27.12.2006,
http://www.nytimes.com/2006/12/27/health/27brain.html
Costs of a Crisis
Diabetics in the Workplace
Confront a
Tangle of Laws
December 26, 2006
The New York Times
By N. R. KLEINFIELD
MINNEAPOLIS — John Steigauf spent more than a
decade fiddling with the innards of those huge United Parcel Service trucks
until an icy day two years ago when the company put him on leave from his
mechanic’s job. A supervisor escorted him off the premises.
His work was good. He hadn’t socked the boss or embezzled money. It had to do
with what was inside him: diabetes.
U.P.S. framed it as a safety issue: Mr. Steigauf’s blood sugar might suddenly
plummet while he tested a truck, causing him to slam into someone.
Mr. Steigauf considered it discrimination, a taint that diabetes can carry. “I
was regarded as a damaged piece of meat,” he said. “It was like, ‘You’re one of
those, and we can’t have one of those.’ ”
With 21 million American diabetics, disputes like this have increasingly rippled
through the workplace:
¶A mortgage loan officer in Oregon was denied permission to eat at her desk to
stanch her sugar fluctuations, and eventually was fired.
¶A Sears lingerie saleswoman in Illinois with nerve damage in her leg quit after
being told she could not cut through a stockroom to reach her department.
¶A worker at a candy company in Wisconsin was fired after asking where he could
dispose of his insulin needles.
In each instance, diabetics contend that they are being blocked by their
employers from the near-normal lives their doctors say are possible. But the
companies say they are struggling, too, with confusion about whether diabetes is
a legitimate disability and with concern about whether it is overly expensive,
hazardous and disruptive to accommodate the illness.
The debate will probably intensify. The number of diabetics in America swelled
by 80 percent in the past decade. Experts say the disease is on its way to
becoming a conspicuous fact of life in the nation’s labor force, raising all
sorts of issues for workers and managers.
Even an outspoken advocate for diabetics like Fran Carpentier, a Type 1 diabetic
and a senior editor at Parade magazine, understands the implications for
business. “Knowing what it’s like to live with the disease hour by hour, day by
day, I wonder if I owned my own company if I would hire someone with diabetes,”
she said. “I’m being bluntly honest. And it kills me to say this.”
Doctors, though, say that with improved medications and methods of self-testing
blood sugar, most diabetics can do almost any job if they properly manage their
illness. Yet myths about the disease persist, advocates say, leading many
companies to shun diabetic employees.
“It’s not all about ignorance, but if I can get rid of ignorance, I can get rid
of a lot of discrimination,” said Shereen Arent, the director of legal advocacy
for the American Diabetes Association.
Part of the confusion is a byproduct of the disease itself, a capricious illness
of elevated, damaging levels of sugar in the blood. Type 1 is a malfunction of
the immune system that usually appears in childhood, while the far more
prevalent Type 2 is closely associated with obesity and inactivity. Many people
with diabetes will face withering complications like blindness, amputations and
heart disease. Others will not.
For some, particularly insulin users prone to the abnormal drops in blood sugar
known as hypoglycemia, the illness can cause dizziness, fainting or muddled
judgment. Doctors, however, say those constitute a tiny number of readily
identifiable cases.
Nonetheless, the risk of plunging blood sugars has fueled a longstanding
reluctance to employ diabetics in jobs like those of truck driver or police
officer, if they are on insulin. Until this summer, the National Fire Protection
Association cautioned against making it too easy for even non-insulin-dependent
diabetics to become firefighters. Now the association recommends an individual
assessment.
Federal law bars diabetics from joining the armed services and prevents
diabetics on insulin from becoming commercial pilots.
Innumerable diabetics, though, are engaged in more mundane jobs uninvolved in
matters of life and death. For these people, secretaries and factory workers and
programmers, a “reasonable accommodation,” like permission to eat at one’s desk
or to be excused from fluctuating shifts, can make the difference in whether
they can function.
When disputes can’t be resolved, the cases often land in court or before the
Equal Employment Opportunity Commission. The commission, which enforces the
Americans With Disabilities Act of 1990, says diabetes-related complaints have
been on the rise, one of the few conditions generally showing an increase in
complaints. Diabetes accounts for nearly 5 percent of the 15,000 annual
allegations that the commission gets under that act, trailing only back
impairment, other orthopedic injuries and depression.
Often the courts are of scant help in bringing clarity. Mr. Steigauf has spent
two years trying to thread his way through the disability discrimination law.
The federal law can be fuzzy, for it mentions no illness or handicap by name but
supplies a legal test under which plaintiffs must usually demonstrate that a
“major life activity,” like walking or vision, is “substantially limited.”
This is easy enough for anyone who has lost sight or a limb. But the
restrictions of diabetes are often invisible. Diabetics thus can find themselves
teetering on a balance beam, needing to prove they are disabled enough to fit
under the law but not so impaired that they can’t do a job.
Judges in nearly identical cases have ruled in completely opposite ways, leaving
diabetics bewildered and businesses unsure what, if anything, they should do.
While some courts, for example, have held that the eating restrictions diabetics
face satisfy the substantial limitation, others have disagreed.
“Usually the battle is over that word, ‘substantially,’ ” said Craig A. Crispin,
an Oregon employment lawyer. “If you say the person is disabled because of the
impact on eating, the other side will say: ‘Hey, look, she’s eating a sandwich.
Where’s the disability?’ ”
Seeking Accommodations
The quarrels are as varied as working life: a musician rejected for a cruise
ship’s cabaret band, baggage handlers and plane cleaners fired by an airline, a
blackjack dealer dismissed by a casino.
The American Diabetes Association fields about 100 calls a month about workplace
tussles like these. Many of them revolve around accommodations, though the
changes sought tend to be modest: predictable hours, a place to test blood,
freedom to snack when sugars get unbalanced.
Companies often cite workplace safety as their paramount concern, though there
is little hard evidence to suggest that diabetics are a risk.
In one case in 2002, ConAgra Foods withdrew a job offer to Rudy Rodriguez at a
Texas baked bean plant after a physical suggested that his Type 2 diabetes was
so out of control that he was a hazard. Mr. Rodriguez had performed fine as an
interim laborer, but the examining doctor declared there was nowhere he could
safely work “outside of a padded room where he could even then fall and break
his neck from dizziness or fainting.”
An appeals court found otherwise and held that ConAgra had violated the law. The
case was settled, and Mr. Rodriguez now works for a printer.
“Some people who have a problem with hypoglycemia should not be doing public
safety-type jobs,” said John W. Griffin Jr., a lawyer from Texas with Type 2
diabetes who handles discrimination cases. “But I guarantee you that that baked
bean factory was not public safety.”
There has been other progress for diabetics: the San Antonio Police Department’s
barring of diabetics on insulin was struck down. Insulin-using diabetics in good
control of their illness can get private pilot’s licenses.
In the lingerie saleswoman’s case, Sears agreed to a consent decree awarding the
woman $150,000 and stipulating that the store train supervisors about disability
discrimination.
Employers, however, prevail in a vast majority of cases (many are settled). It
is hard even to get lawyers to pursue complaints since prejudice is tricky to
prove. Establishing discrimination has become harder since 1999, when the
Supreme Court held that if a disability can be corrected with medicine or things
like prostheses, it is not necessarily protected. Advocates for the disabled say
the ruling warped the intent of Congress.
Ruth Colker, a law professor at Ohio State University who studies disability
discrimination, said that very few working people with diabetes now find
themselves guarded by the law.
Judges, in fact, have deemed these diabetics not disabled: a Maine store manager
who had trouble walking because of poor circulation, and a New York security
guard without vision in one eye and declining vision in the other who had four
episodes of hypoglycemia in two years.
In some instances, employers have said they took action against an employee
because of diabetes, but the court still found that the worker was not disabled
and threw out the case.
“There’s not a remedy for every wrong,” said David A. Copus, a lawyer who
represents employers and specializes in disability issues. “There are employers
who don’t like ugly people. They’re not protected by the law.”
Mr. Copus said he was not unsympathetic. His father had diabetes; it was very
debilitating.
Wary of bad outcomes, many diabetics conceal their illness on the job. Brian T.
McMahon, a professor at Virginia Commonwealth University who studies workplace
discrimination, said: “You get to the question of whether or not to disclose you
have diabetes. Most people opt not to, for they fear: Am I inviting more
trouble?”
Figuring Employer Costs
There was a time, four or five decades ago, when you wouldn’t find one diabetic
on the entire floor of a factory. Now, Type 2 diabetics are commonplace. It is
not only the ascendancy of the disease, but also the fact that a condition once
considered a corollary to old age is striking people sooner, catching them long
before retirement. And this comes as companies are already struggling to balance
productivity in the workplace with soaring medical costs.
To understand the brutal math of diabetes, all a business has to do is consult
the Web site diabetesatwork.org, set up by the government to furnish advice on
addressing diabetes in the workplace. One of its tools is a calculator that uses
rough assumptions to suggest what costs might be involved.
Businesses plug in the number of employees, the tally is multiplied by 8.2
percent (a slightly dated national prevalence rate for diabetes), then that
figure is multiplied by $13,243, an estimate for yearly medical costs of a
diabetic. Voilà: the price tag of diabetes. It is a burden more than five times
that of workers without diabetes.
Ron Z. Goetzel, a vice president for Thomson Medstat, which analyzes health care
costs for businesses, said that if absenteeism and productivity losses are
added, diabetes ranks third among major conditions as an economic cost to
employers, after heart disease and hypertension.
Companies have only started to reckon with this, and with the disease’s
ancillary concerns. Even if advocates say safety is rarely a factor, companies
argue they cannot take chances with some types of workers, like school bus
drivers or even pizza deliverers.
Concessions may seem small — for example, granting a bank teller more frequent
breaks — but many employers contend that if rules bend for one person, then that
breeds resentment among other employees. Co-workers cannot see the diabetes, and
if an employer gives preferential treatment to a diabetic worker, it cannot
legally tell other workers it is because of the diabetes. Companies feel that
indulging all diabetics trivializes the meaning of disability and of fairness.
“It comes down to how many extra points do I give you,” said William J. Kilberg,
a Washington employment lawyer. “Why is everybody a victim?”
He said too many people who are not disabled demand special favors. “I mean, I
wear eyeglasses,” he said.
In addition to the threat of a suit under federal or state disability law,
businesses must grapple with the Family and Medical Leave Act, which requires
them to grant unpaid leave to ill workers. That can create scheduling
difficulties.
“This whole area gets complicated, because the medical leave act can mean
employees absent from the workplace for extended periods,” said Stephen A.
Bokat, general counsel for the United States Chamber of Commerce. “That bothers
employers even more than an employee needing a half-hour during the day to
administer some insulin.”
Health care consultants urge companies to become proactive, try to use tools
like wellness programs to forestall diabetes’ claim on their workers and install
disease management programs to improve existing cases. And many do. Companies
dangle $100 incentives (though more often a mug or a T-shirt) if an employee
submits to a health assessment or accepts a phone call from a health coach.
But getting participation is hard. And the economic worth of these undertakings
to a business is difficult to gauge with a progressive disease like diabetes.
Sometimes the evident benefits of in-house health programs are years down the
road, when an employee a business invested in may well be working elsewhere.
When Safety Comes First
John Steigauf’s fellow mechanics called him “Flunky.” The name caught on after
Mr. Steigauf gave advice to a prickly supervisor, who thundered, “I’m not going
to let some flunky mechanic tell me how to do my job.” Mr. Steigauf, 47, wanted
it embroidered onto his uniform. Supervisors said forget it.
He joined United Parcel in Minneapolis in 1991, turning wrenches from the start.
Five years ago, he learned that he had Type 2 diabetes. Though it often goes
with being overweight, he had a wide receiver’s build: 6-foot-2, 193 pounds. But
his mother had diabetes.
He was put on pills and watched his diet, abandoning his cherished hot
chocolate. Yet his blood sugar remained high. If it didn’t drop, he would need
insulin. That sent fear pumping through him.
U.P.S. requires mechanics, like its interstate drivers, to hold a commercial
driver’s license and to be cleared to drive out of state so they can road-test
trucks. In reality, mechanics could go weeks without leaving the yard, but those
were the rules. And at the time, federal officials did not grant interstate
licenses to insulin-using diabetics.
They did dispense a few exemptions. But Mr. Steigauf, who started using insulin
only in 2004, stood no chance. The requirement was to have driven safely while
on insulin for three years.
So Mr. Steigauf tried starving himself, slicing off 30 pounds. It made him
crabby, fatigued and so thin “that if I stuck my tongue out, I looked like a
zipper.”
His blood sugars did not budge. But after he took insulin, his diabetes settled
down almost at once.
Later in 2004, he had to have a physical to renew his commercial license, a test
he could no longer pass. Minnesota did not have the three-year rule, and since
he had never had a hypoglycemic episode or other problems, Mr. Steigauf got an
exemption to drive within the state. Given that he didn’t even leave the county
while road-testing trucks, he hoped that would be good enough.
It wasn’t. For consistency, U.P.S. wanted all its mechanics to be certified to
drive from state to state, whether they needed to or not. So Mr. Steigauf was
sent home on disability, even though he felt fine.
Norman Black, a U.P.S. spokesman, said the company had always intended to find
Mr. Steigauf another position. He said the company did not discriminate but was
passionate about safety.
Studies tracking accident rates of diabetics are inconclusive; some indicate
worse outcomes, others don’t. But either way, Mr. Steigauf’s direct supervisor
at the time, Dan Welke, said he thought the company had gone too far, that
somebody else could road-test the trucks repaired by Mr. Steigauf.
“It just seemed ridiculous,” Mr. Welke said.
Mr. Steigauf also had trouble making sense of this. When his diabetes raged out
of control, it had been all right to fix trucks. Now, with the illness under
control, it wasn’t.
When he spoke to someone in human resources, Mr. Steigauf said he was told that
he would never come back. The notion that he was a human tinderbox punctuated
his interactions. “I’d be told that I could pull a tractor up to a fuel pump and
pass out and the thing would explode,” he said. “I was like a grenade and was
going to kill people.”
At home in Bloomington, Minn., where Mr. Steigauf’s tidy ranch is bordered by a
soupçon of lawn, he, his wife, Dawn, their twin daughters and their severely
autistic son struggled on a weekly disability check of $431, about half his old
pay.
To keep going, he did odd jobs. Friends at U.P.S. dug into their pockets. The
family cut one another’s hair and skipped sending Christmas cards.
Finally, Mr. Steigauf asked U.P.S. to excuse him from interstate driving as a
“reasonable accommodation” under disability law. After all, he had left the
state for U.P.S. only once in 13 years. Some of his colleagues, he found out,
did not even have commercial driver’s licenses.
But U.P.S. refused, saying he was not disabled under discrimination law, and
thus not entitled to an accommodation.
So, in early 2005, he filed a complaint with the Equal Employment Opportunity
Commission and waited, interned in his house, as if he had evaporated — all the
while collecting disability from a company that said he was not disabled.
After seven months, U.P.S. offered him a lower-paying job, fixing trailers. He
could not touch an engine or drive. But he took it while he battled for his
former position.
Then he had some luck. Federal officials changed their rules on interstate
licenses and Mr. Steigauf qualified for a waiver, allowing him to return to his
old job. He is expected to start within a month or so.
This fall, the E.E.O.C. concluded that he had been discriminated against and
that U.P.S. owed him relief. U.P.S. said it would contest the decision.
Mr. Steigauf is still bitter, not toward U.P.S. itself, but toward the way he
believes it treats diabetics. Even now, he feels singled out.
“I’ve had unloaders at work say to me, ‘Are you that diabetic guy?’ ” he said.
“I don’t know what they mean. Nothing? Or, ‘You shouldn’t be working here’?”
To keep his exemption, he must obey a complicated protocol to show the Federal
Motor Carrier Safety Administration that he remains fit. Every three months, he
has to report how much he drives and his sugar levels. If he has an accident,
even if someone rams his car while he’s in a movie, he has two days to alert the
government. He has seven days to let the government know if he has a new car or
a new phone number.
“The exemption adds to the discrimination,” he said one afternoon at home. “It
constantly reminds me that I’m different.”
His children skittered through the room.
“You become a show dog,” he said. “I fix engines. I don’t want to be a show
dog.”
Diabetics in the Workplace Confront a Tangle of Laws, NYT, 26.12.2006,
http://www.nytimes.com/2006/12/26/health/26workplace.html
The Doctor’s World
The Man on the Table
Devised the Surgery
December 25, 2006
The New York Times
By LAWRENCE K. ALTMAN
In late afternoon last Dec. 31, Dr. Michael E.
DeBakey, then 97, was alone at home in Houston in his study preparing a lecture
when a sharp pain ripped through his upper chest and between his shoulder
blades, then moved into his neck.
Dr. DeBakey, one of the most influential heart surgeons in history, assumed his
heart would stop in a few seconds.
“It never occurred to me to call 911 or my physician,” Dr. DeBakey said, adding:
“As foolish as it may appear, you are, in a sense, a prisoner of the pain, which
was intolerable. You’re thinking, What could I do to relieve myself of it. If it
becomes intense enough, you’re perfectly willing to accept cardiac arrest as a
possible way of getting rid of the pain.”
But when his heart kept beating, Dr. DeBakey suspected that he was not having a
heart attack. As he sat alone, he decided that a ballooning had probably
weakened the aorta, the main artery leading from the heart, and that the inner
lining of the artery had torn, known as a dissecting aortic aneurysm.
No one in the world was more qualified to make that diagnosis than Dr. DeBakey
because, as a younger man, he devised the operation to repair such torn aortas,
a condition virtually always fatal. The operation has been performed at least
10,000 times around the world and is among the most demanding for surgeons and
patients.
Over the past 60 years, Dr. DeBakey has changed the way heart surgery is
performed. He was one of the first to perform coronary bypass operations. He
trained generations of surgeons at the Baylor College of Medicine; operated on
more than 60,000 patients; and in 1996 was summoned to Moscow by Boris Yeltsin,
then the president of Russia, to aid in his quintuple heart bypass operation.
Now Dr. DeBakey is making history in a different way — as a patient. He was
released from Methodist Hospital in Houston in September and is back at work. At
98, he is the oldest survivor of his own operation, proving that a healthy man
of his age could endure it.
“He’s probably right out there at the cutting edge of a whole generation of
people in their 90s who are going to survive” after such medical ordeals, one of
his doctors, Dr. James L. Pool, said.
But beyond the medical advances, Dr. DeBakey’s story is emblematic of the
difficulties that often accompany care at the end of life. It is a story of
debates over how far to go in treating someone so old, late-night disputes among
specialists about what the patient would want, and risky decisions that, while
still being argued over, clearly saved Dr. DeBakey’s life.
It is also a story of Dr. DeBakey himself, a strong-willed pioneer who at one
point was willing to die, concedes he was at times in denial about how sick he
was and is now plowing into life with as much zest and verve as ever.
But Dr. DeBakey’s rescue almost never happened.
He refused to be admitted to a hospital until late January. As his health
deteriorated and he became unresponsive in the hospital in early February, his
surgical partner of 40 years, Dr. George P. Noon, decided an operation was the
only way to save his life. But the hospital’s anesthesiologists refused to put
Dr. DeBakey to sleep because such an operation had never been performed on
someone his age and in his condition. Also, they said Dr. DeBakey had signed a
directive that forbade surgery.
As the hospital’s ethics committee debated in a late-night emergency meeting on
the 12th floor of Methodist Hospital, Dr. DeBakey’s wife, Katrin, barged in to
demand that the operation begin immediately.
In the end, the ethics committee approved the operation; an anesthesiology
colleague of Dr. DeBakey’s, who now works at a different hospital, agreed to put
him to sleep; and the seven-hour operation began shortly before midnight on Feb.
9. “It is a miracle,” Dr. DeBakey said as he sat eating dinner in a Houston
restaurant recently. “I really should not be here.”
The costs of Dr. DeBakey’s care easily exceeded $1 million. Methodist Hospital
and his doctors say they have not charged Dr. DeBakey. His hospitalizations were
under pseudonyms to help protect his privacy, which could make collecting
insurance difficult. Methodist Hospital declined to say what the costs were or
discuss the case further. Dr. DeBakey says he thinks the hospital should not
have been secretive about his illness.
Dr. DeBakey’s doctors acknowledge that he got an unusually high level of care.
But they said that they always tried to abide by a family’s wishes and that they
would perform the procedure on any patient regardless of age, if the patient’s
overall health was otherwise good.
Dr. DeBakey agreed to talk, and permitted his doctors to talk, because of a
professional relationship of decades with this reporter, who is also a
physician, and because he wanted to set the record straight for the public about
what happened and explain how a man nearly 100 years old could survive.
A Preliminary Diagnosis
As Dr. DeBakey lay on the couch alone that night, last New Year’s Eve, he
reasoned that a heart attack was unlikely because periodic checkups had never
indicated he was at risk. An aortic dissection was more likely because of the
pain, even though there was no hint of that problem in a routine echocardiogram
a few weeks earlier.
Mrs. DeBakey and their daughter, Olga, had left for the beach in Galveston, but
turned back because of heavy traffic. They arrived home to find Dr. DeBakey
lying on the couch. Not wanting to alarm them, he lied and said he had fallen
asleep and awakened with a pulled muscle.
“I did not want Katrin to be aware of my self-diagnosis because, in a sense, I
would be telling her that I am going to die soon,” he said.
An anxious Mrs. DeBakey called two of her husband’s colleagues: Dr. Mohammed
Attar, his longtime physician, and Dr. Matthias Loebe, who was covering for Dr.
Noon. They came to the house quickly and became concerned because Dr. DeBakey
had been in excellent health. After listening to him give a more frank account
of his pain, they shared his suspicion of an aortic dissection.
Dr. DeBakey and his doctors agreed that for a firm diagnosis he would need a CT
scan and other imaging tests, but he delayed them until Jan. 3.
The tests showed that Dr. DeBakey had a type 2 dissecting aortic aneurysm,
according to a standard classification system he himself devised years earlier.
Rarely did anyone survive that without surgery.
Still, Dr. DeBakey says that he refused admission to Methodist Hospital, in part
because he did not want to be confined and he “was hopeful that this was not as
bad as I first thought.” He feared the operation that he had developed to treat
this condition might, at his age, leave him mentally or physically crippled.
“I’d rather die,” he said.
Over the years, he had performed anatomically perfect operations on some
patients who nevertheless died or survived with major complications. “I was
trying to avoid all that,” he said.
Instead, he gambled on long odds that his damaged aorta would heal on its own.
He chose to receive care at home. For more than three weeks, doctors made
frequent house calls to make sure his blood pressure was low enough to prevent
the aorta from rupturing. Around the clock, nurses monitored his food and drink.
Periodically, he went to Methodist Hospital for imaging tests to measure the
aneurysm’s size.
On Jan. 6, he insisted on giving the lecture he had been preparing on New Year’s
Eve to the Academy of Medicine, Engineering and Science of Texas, of which he is
a founding member. The audience in Houston included Nobel Prize winners and
Senator Kay Bailey Hutchison.
Mrs. DeBakey stationed people around the podium to catch her husband if he
slumped. Dr. DeBakey looked gray and spoke softly, but finished without
incident. Then he listened to another lecture — which, by coincidence, was about
the lethal dangers of dissecting aneurysms.
Dr. DeBakey, a master politician, said he could not pass up a chance to chat
with the senator. He attended the academy luncheon and then went home.
In providing the extraordinary home care, the doctors were respecting the wishes
of Dr. DeBakey and their actions reflected their awe of his power.
“People are very scared of him around here,” said Dr. Loebe, the heart surgeon
who came to Dr. DeBakey’s home on New Year’s Eve. “He is the authority. It is
very difficult to stand up and tell him what to do.”
But as time went on, the doctors could not adequately control Dr. DeBakey’s
blood pressure. His nutrition was poor. He became short of breath. His kidneys
failed. Fluid collected in the pericardial sac covering his heart, suggesting
the aneurysm was leaking.
Dr. DeBakey now says that he was in denial. He did not admit to himself that he
was getting worse. But on Jan. 23, he yielded and was admitted to the hospital.
Tests showed that the aneurysm was enlarging dangerously; the diameter increased
to 6.6 centimeters on Jan. 28, up from 5.2 centimeters on Jan. 3. Dr. Noon said
that when he and other doctors showed Dr. DeBakey the scans and recommended
surgery, Dr. DeBakey said he would re-evaluate the situation in a few days.
By Feb. 9, with the aneurysm up to 7.5 centimeters and Dr. DeBakey unresponsive
and near death, a decision had to be made.
“If we didn’t operate on him that day that was it, he was gone for sure,” Dr.
Noon said.
At that point, Dr. DeBakey was unable to speak for himself. The surgeons
gathered and decided they should proceed, despite the dangers. “We were doing
what we thought was right,” Dr. Noon said, adding that “nothing made him a
hopeless candidate for the operation except for being 97.” All family members
agreed to the operation.
Dr. Bobby R. Alford, one of Dr. DeBakey’s physicians and a successor as
chancellor of Baylor College of Medicine, said the doctors had qualms. “We could
have walked away,” he said.
He and Dr. Noon discussed the decision several times. “We recognized the
condemnation that could occur,” Dr. Alford said. “The whole surgical world would
come down on us for doing something stupid, which it might have seemed to people
who were not there.”
Surgery would be enormously risky and unlikely to offer clear-cut results —
either a full recovery or death, Dr. Noon and his colleagues told Mrs. DeBakey,
Olga, sons from a first marriage, and Dr. DeBakey’s sisters, Lois and Selma. The
doctors said Dr. DeBakey might develop new ailments and need dialysis and a
tracheostomy to help his breathing. They said the family’s decision could
inflict prolonged suffering for all involved.
Olga and she “prayed a lot,” said Mrs. DeBakey, who is from Germany. “We had a
healer in Europe who advised us that he will come through it. That helped us.”
Then things got more complicated.
A Refusal to Treat
At that point the Methodist Hospital anesthesiologists adamantly refused to
accept Dr. DeBakey as a patient. They cited a standard form he had signed
directing that he not be resuscitated if his heart stopped and a note in the
chart saying he did not want surgery for the aortic dissection and aneurysm.
They were concerned about his age and precarious physical condition.
Dr. Alford, the 72-year-old chancellor, said he was stunned by the refusal, an
action he had never seen or heard about in his career.
Dr. Noon said none of the anesthesiologists had been involved in Dr. DeBakey’s
care, yet they made a decision based on grapevine information without reading
his medical records. So he insisted that the anesthesiologists state their
objections directly to the DeBakey family.
Mrs. DeBakey said the anesthesiologists feared that Dr. DeBakey would die on the
operating table and did not want to become known as the doctors who killed him.
Dr. Joseph J. Naples, the hospital’s chief anesthesiologist, did not return
repeated telephone calls to his office for comment.
Around 7 p.m., Mrs. DeBakey called Dr. Salwa A. Shenaq, an anesthesiologist
friend who had worked with Dr. DeBakey for 22 years at Methodist Hospital and
who now works at the nearby Michael E. DeBakey Veterans Affairs Medical Center.
Dr. Shenaq rushed from home. When she arrived, she said, Dr. Naples told her
that he and his staff would not administer anesthesia to Dr. DeBakey. She said
that a medical staff officer, whom she declined to name, warned her that she
could be charged with assault if she touched Dr. DeBakey. The officer also told
Dr. Shenaq that she could not give Dr. DeBakey anesthesia because she did not
have Methodist Hospital privileges. She made it clear that she did, she said.
Administrators, lawyers and doctors discussed the situation, in particular the
ambiguities of Dr. DeBakey’s wishes. Yes, Dr. Pool had written on his chart that
Dr. DeBakey said he did not want surgery for a dissection. But Dr. Noon and the
family thought the note in the chart no longer applied because Dr. DeBakey’s
condition had so deteriorated and his only hope was his own procedure.
“They were going back and forth,” Dr. Shenaq said. “One time, they told me go
ahead. Then, no, we cannot go ahead.”
To fulfill its legal responsibilities, Methodist Hospital summoned members of
its ethics committee, who arrived in an hour. They met with Dr. DeBakey’s
doctors in a private dining room a few yards from Dr. DeBakey’s room, according
to five of his doctors who were present.
Their patient was a man who had always been in command. Now an unresponsive Dr.
DeBakey had no control over his own destiny.
The ethics committee representatives wanted to follow Texas law, which, in part,
requires assurance that doctors respect patient and family wishes.
Each of Dr. DeBakey’s doctors had worked with him for more than 20 years. One,
Dr. Pool, said they felt they knew Dr. DeBakey well enough to answer another
crucial question from the ethics committee: As his physicians, what did they
believe he would choose for himself in such a dire circumstance if he had the
ability to make that decision?
Dr. Noon said that Dr. DeBakey had told him it was time for nature to take its
course, but also told him that the doctors had “to do what we need to do.”
Members of Dr. DeBakey’s medical team said they interpreted the statements
differently. Some thought he meant that they should do watchful waiting, acting
only if conditions warranted; others thought it meant he wanted to die.
The question was whether the operation would counter Dr. DeBakey’s wishes
expressed in his signed “do not resuscitate” order. Some said that everything
Dr. DeBakey did was for his family. And the family wanted the operation.
After the committee members had met for an hour, Mrs. DeBakey could stand it no
longer. She charged into the room.
“My husband’s going to die before we even get a chance to do anything — let’s
get to work,” she said she told them.
The discussion ended. The majority ruled in a consensus without a formal vote.
No minutes were kept, the doctors said.
“Boy, when that meeting was over, it was single focus — the best operation, the
best post-operative care, the best recovery we could give him,” Dr. Pool said.
The Operation
As the ethics committee meeting ended about 11 p.m. on Feb. 9, the doctors
rushed to start Dr. DeBakey’s anesthesia.
The operation was to last seven hours.
For part of that time, Dr. DeBakey’s body was cooled to protect his brain and
other organs. His heart was stilled while a heart-lung bypass machine pumped
oxygen-rich blood through his body. The surgeons replaced the damaged portion of
Dr. DeBakey’s aorta with a six- to eight-inch graft made of Dacron, similar to
material used in shirts. The graft was the type that Dr. DeBakey devised in the
1950s.
Afterward, Dr. DeBakey was taken to an intensive care unit.
Some doctors were waiting for Dr. DeBakey to die during the operation or soon
thereafter, Dr. Noon said. “But he just got better.”
As feared, however, his recovery was stormy.
Surgeons had to cut separate holes into the trachea in his neck and stomach to
help him breathe and eat. He needed dialysis because of kidney failure. He was
on a mechanical ventilator for about six weeks because he was too weak to
breathe on his own. He developed infections. His blood pressure often fell too
low when aides lifted him to a sitting position. Muscle weakness left him unable
to stand.
For a month, Dr. DeBakey was in the windowless intensive care unit, sometimes
delirious, sometimes unresponsive, depending in part on his medications. The
doctors were concerned that he had suffered severe, permanent brain damage. To
allow him to tell day from night and lift his spirits, the hospital converted a
private suite into an intensive care unit.
Some help came from unexpected places. On Sunday, April 2, Dr. William W. Lunn,
the team’s lung specialist, took his oldest daughter, Elizabeth, 8, with him
when he made rounds at the hospital and told her that a patient was feeling
blue. While waiting, Elizabeth drew a cheery picture of a rainbow, butterflies,
trees and grass and asked her father to give it to the patient. He did.
“You should have seen Dr. DeBakey’s eyes brighten,” Dr. Lunn said. Dr. DeBakey
asked to see Elizabeth, held her hand and thanked her.
“At that point, I knew he was going to be O.K.,” Dr. Lunn said.
Dr. DeBakey was discharged on May 16. But on June 2, he was back in the
hospital.
“He actually scared us because his blood pressure and heart rate were too high,
he was gasping for breath” and he had fluid in his lungs, Dr. Lunn said.
But once the blood pressure was controlled with medicine, Dr. DeBakey began to
recover well.
The Aftermath
At times, Dr. DeBakey says he played possum with the medical team, pretending to
be asleep when he was listening to conversations.
On Aug. 21, when Dr. Loebe asked Dr. DeBakey to wake up, and he did not, Dr.
Loebe announced that he had found an old roller pump that Dr. DeBakey devised in
the 1930s to transfuse blood. Dr. DeBakey immediately opened his eyes. Then he
gave the doctors a short lecture about how he had improved it over existing
pumps.
As he recovered and Dr. DeBakey learned what had happened, he told his doctors
he was happy they had operated on him. The doctors say they were relieved
because they had feared he regretted their decision.
“If they hadn’t done it, I’d be dead,” he said.
The doctors and family had rolled the dice and won.
Dr. DeBakey does not remember signing an order saying not to resuscitate him and
now thinks the doctors did the right thing. Doctors, he said, should be able to
make decisions in such cases, without committees.
Throughout, Dr. DeBakey’s mental recovery was far ahead of his physical
response.
When Dr. DeBakey first became aware of his post-operative condition, he said he
“felt limp as a rag” and feared he was a quadriplegic. Kenneth Miller and other
physical therapists have helped Dr. DeBakey strengthen his withered muscles.
“There were times where he needed a good bit of encouragement to participate,”
Mr. Miller said. “But once he saw the progress, he was fully committed to what
we were doing.”
Now he walks increasingly long distances without support. But his main means of
locomotion is a motorized scooter. He races it around corridors, sometimes
trailed by quick-stepping doctors of all ages.
Dr. DeBakey said he hoped to regain the stamina to resume traveling, though not
at his former pace.
Dr. William L. Winters Jr., a cardiologist on Dr. DeBakey’s team, said: “I am
impressed with what the body and mind can do when they work together. He
absolutely has the desire to get back to where he was before. I think he’ll come
close.”
Already, Dr. DeBakey is back working nearly a full day.
“I feel very good,” he said Friday. “I’m getting back into the swing of things.”
The
Man on the Table Devised the Surgery, NYT, 26.12.2006,
http://www.nytimes.com/2006/12/25/health/25surgeon.html
Editorial
A Big Drop in Breast Cancer
December 16, 2006
The New York Times
The sharp drop in breast cancer rates reported
this week is astonishingly good news. It is the first major reduction in the
incidence of a malignancy that strikes more than 200,000 American women every
year — and kills some 40,000 annually.
Researchers from the M. D. Anderson Cancer Center in Houston and other
institutions reported that the incidence of all types of breast cancer fell a
stunning 7 percent in 2003 — the latest year for which statistics are available
— from the year before. This was the first such decline after persistent rises
for several decades and a leveling off from 1998 to 2002. The researchers
estimate that 14,000 fewer women were diagnosed with breast cancer in 2003 than
in the year before.
The most plausible explanation is that women by the millions abandoned or
sharply cut back their use of hormone therapy. For many years hormones — which
have been widely used to treat the symptoms of menopause — had also been hyped
by the pharmaceutical industry as an elixir to ward off the ravages of aging.
Overly enthusiastic doctors also championed hormone therapy as a way to prevent
or mitigate heart disease, Alzheimer’s, severe depression and urinary
incontinence — none of which turned out to be true.
But in mid-2002, a study of the effects of hormones on thousands of women had to
be halted after it became clear that prolonged use of a popular hormone
combination caused an increase in breast cancer, heart attacks, strokes and
blood clots.
Women abandoned the hormone pills in droves, and almost immediately, the
incidence of breast cancer began to fall. As Gina Kolata reported in yesterday’s
Times, rates of the most common form of breast cancer — tumors that are fueled
by estrogen — dropped a startling 15 percent from August 2002 to December 2003.
The researchers hypothesize that tiny tumors in the breast, when deprived of the
hormones that fueled them, stopped growing or at least grew more slowly, leaving
them too small to be detected on mammograms. It is also possible that, with
their hormones cut off, some tumors shrank or even disappeared. Other factors,
like a slight dip in mammography screening to detect tumors and use of drugs
that are known or thought to slow breast cancer, might have played a small role
in reducing the numbers, but they were deemed too inconsequential to explain the
results.
The great unknown is what will happen in the future. If tumor growth was simply
slowed, not stopped, the tumors may become detectable as time goes on. But
hormone therapy has continued to decline, so the drop in breast cancer is apt to
continue. A study in California found that a sharp decrease in breast cancer
incidence in 2003 was followed by a slower but continued drop in 2004, a
harbinger, perhaps. of what national statistics will show next year.
Further analyses will be needed to identify all possible reasons for the decline
of breast cancer incidences. If the hypothesis holds up that the drop in hormone
use is the main cause, as seems likely, it should persuade even more women to
curb their use except when absolutely necessary. Meanwhile, breast cancer
incidence will remain high, underscoring the need for more ways to prevent this
dreaded disease.
A Big
Drop in Breast Cancer, NYT, 16.12.2006,
http://www.nytimes.com/2006/12/16/opinion/16sat1.html
Op-Ed Contributor
A Second Look at Death
December 15, 2006
The New York Times
By FRANCIS L. DELMONICO
Boston
RECENTLY, a 15-year-old girl lay in a hospital here with brain injuries from a
car accident. The trauma and bleeding were extensive, and nothing could be done
to save her life. Her parents agreed that she should be taken off the
respirator, and then requested that she be an organ donor.
However, this child was not brain dead, the usual first step in organ donation.
Although most of her brain had shut down, the brain stem, which manages
unconscious functions like breathing, was working enough to set off the
respirator sporadically.
So the organ donation was managed in another way. After the respirator was
removed, and after the girl’s heart had stopped beating and she had quit
breathing, her doctors pronounced her dead by cardiorespiratory criteria. Her
heart was not suitable for donation — there was no way to predict that it would
resume beating after transplantation. But the doctors were able to arrange for
her liver and kidneys to be transplanted.
Had the girl died at another hospital, this might not have happened, because
despite evidence that this kind of donation works well, some doctors and
hospital administrators remain reluctant to procure organs from patients after
cardiac death.
Thanks to a redoubling of effort to look at every death in the hospital as a
potential occasion for donation, the number of deceased donors has risen 23
percent since 2003, to about 700 per month, according to the federal Health
Resources and Services Administration. Still, many patients on organ waiting
lists die every day. And the demand has led to more donations from living
donors, who then face medical risks of their own.
If donation after cardiac death were pursued as diligently as donation after
brain death, the number of organs available for transplant could rise
significantly.
Donation after cardiac death was once the only form of transplantation from
deceased donors — until 40 years ago, when doctors began using cessation of
brain activity as a way to determine death. Brain activity is a preferable
measure, because it enables the deceased patient to remain attached to a
respirator until moments before organs are removed. This ensures that the
donor’s heart continues to beat, supplying all the organs with oxygen and
nutrients.
Soon after adopting the brain death protocol, doctors largely abandoned donation
after cardiac death, on the assumption that the organs would fare poorly after
transplant. But lungs and livers usually do remain viable after cardiac death.
And kidneys, if promptly transplanted, do just as well as those recovered from
brain-dead donors, according to the United Network for Organ Sharing, a
nonprofit organization that manages the transplant network in the United States.
Some doctors have objected to donation after cardiac death because they are
uneasy about taking organs too soon after a patient’s heart stops. But an
extensive Institute of Medicine study in the late 1990s confirmed that donation
after cardiac death adheres to the fundamental dead donor rule: that the
recovery of organs is not the cause of death.
The Institute also laid down three key guidelines for donation after cardiac
death: proper consent should be obtained, the donor should be ruled dead by a
doctor other than the transplant surgeon, and the ruling should be delayed five
minutes after the patient’s heart has stopped — to ensure that it will not
revive spontaneously.
The propriety of donation after cardiac death is so well established and its
potential to ease organ shortages is so great that the Health Resources and
Services Administration has deemed it an important goal for the nation. In
January, the Joint Commission on Accreditation of Healthcare Organizations,
which sets standards for medical practice in most American hospitals, will
require protocols that enable the practice. But the group has made a key
exception: a hospital that is unfamiliar and uncomfortable with the practice may
opt out. This makes it unlikely that the protocols will be put to widespread
use.
Individual hospitals should not decide that accepted practices of medicine are
improper or unimportant. As long as a segment of the medical community stands in
the way of donation after cardiac death, lives will be lost unnecessarily. And
families of potential donors, at a time of enormous grief, will be deprived of
the comfort of knowing that their loved ones’ organs can be used to save lives.
Francis L. Delmonico is a transplant surgeon at Massachusetts General
Hospital and the medical director of the New England Organ Bank.
A
Second Look at Death, NYT, 15.12.2006,
http://www.nytimes.com/2006/12/15/opinion/15delmonico.html
Circumcision Halves H.I.V. Risk,
U.S.
Agency Finds
December 14, 2006
The New York Times
By DONALD G. McNEIL Jr.
Circumcision appears to reduce a man’s risk of
contracting AIDS from heterosexual sex by half, United States government health
officials said yesterday, and the directors of the two largest funds for
fighting the disease said they would consider paying for circumcisions in
high-risk countries.
The announcement was made by officials of the National Institutes of Health as
they halted two clinical trials, in Kenya and Uganda, on the ground that not
offering circumcision to all the men taking part would be unethical. The success
of the trials confirmed a study done last year in South Africa.
AIDS experts immediately hailed the finding. “This is very exciting news,” said
Daniel Halperin, an H.I.V. specialist at the Harvard Center for Population and
Development, who has argued that circumcision slows the spread of AIDS in the
parts of Africa where it is common.
In an interview from Zimbabwe, he added, “I have no doubt that as word of this
gets around, millions of African men will want to get circumcised, and that will
save many lives.”
Uncircumcised men are thought to be more susceptible because the underside of
the foreskin is rich in Langerhans cells, sentinel cells of the immune system,
which attach easily to the human immunodeficiency virus, which causes AIDS. The
foreskin also often suffers small tears during intercourse.
But experts also cautioned that circumcision is no cure-all. It only lessens the
chances that a man will catch the virus; it is expensive compared to condoms,
abstinence or other methods; and the surgery has serious risks if performed by
folk healers using dirty blades, as often happens in rural Africa.
Circumcision is “not a magic bullet, but a potentially important intervention,”
said Dr. Kevin M. De Cock, director of H.I.V./AIDS for the World Health
Organization.
Sex education messages for young men need to make it clear that “this does not
mean that you have an absolute protection,” said Dr. Anthony S. Fauci, an AIDS
researcher and director of the National Institute of Allergy and Infectious
Diseases.
Circumcision should be used with other prevention methods, he said, and it does
nothing to prevent spread by anal sex or drug injection, ways in which the virus
commonly spreads in the United States.
The two trials, conducted by researchers from universities in Illinois,
Maryland, Canada, Uganda and Kenya, involved nearly 3,000 heterosexual men in
Kisumu, Kenya, and nearly 5,000 in Rakai, Uganda. None were infected with H.I.V.
They were divided into circumcised and uncircumcised groups, given safe sex
advice (although many presumably did not take it), and retested regularly.
The trials were stopped this week by the N.I.H. Data Safety and Monitoring Board
after data showed that the Kenyan men had a 53 percent reduction in new H.I.V.
infection. Twenty-two of the 1,393 circumcised men in that study caught the
disease, compared with 47 of the 1,391 uncircumcised men.
In Uganda, the reduction was 48 percent.
Those results echo the finding of a trial completed last year in Orange Farm, a
township in South Africa, financed by the French government, which demonstrated
a reduction of 60 percent among circumcised men.
The two largest agencies dedicated to fighting AIDS said they would now be
willing to pay for circumcisions, which they have not before because there was
too little evidence that it worked.
Dr. Richard G. A. Feachem, executive director of the Global Fund to Fight AIDS,
Tuberculosis and Malaria, which has almost $5 billion in pledges, said in a
television interview that if a country submitted plans to conduct sterile
circumcisions, “I think it’s very likely that our technical panel would approve
it.”
Dr. Mark Dybul, executive director of President Bush’s $15 billion Emergency
Plan for AIDS Relief, said in a statement that his agency “will support
implementation of safe medical male circumcision for H.I.V./AIDS prevention” if
world health agencies recommend it.
He also warned that it was only one new weapon in the fight, adding, “Prevention
efforts must reinforce the A.B.C. approach — abstain, be faithful, and correct
and consistent use of condoms.”
Researchers have long noted that parts of Africa where circumcision is common —
particularly the Muslim countries of West Africa — have much lower AIDS rates,
while those in southern Africa, where circumcision is rare, have the highest.
But drawing conclusions was always confounded by other regional factors, like
strict Shariah law in some Muslim areas, rape and genocide in East Africa,
polygamy, rites that require widows to have sex with a relative, patronage of
prostitutes by miners, and men’s insistence on dangerous “dry sex” — with the
woman’s vaginal walls robbed of secretions with desiccating herbs.
Outside Muslim regions, circumcision is spotty. In South Africa, for example,
the Xhosa people circumcise teenage boys, while Zulus do not. AIDS is common in
both tribes.
Nelson Mandela’s autobiography, “Long Walk to Freedom,” contains an unnerving
but hilarious account of his own Xhosa circumcision, by spear blade, as a
teenager. Although he was supposed to shout, “I am a man!” he grimaced in pain,
he wrote.
But not all initiation ceremonies are laughing matters. Every year, some South
African teenagers die from infections, and the use of one blade on many young
men may help spread AIDS.
In recent years, as word has spread that circumcision might be protective, many
southern African men have sought it out. A Zambian hospital offered $3
circumcisions last year, and Swaziland trained 60 doctors to do them for $40
after waiting lists at its national hospital grew.
“Private practitioners also do it,” Dr. Halperin said. “In some places, it’s
$20; in others, much more. Lots of the wealthy elite have already done it. It
prevents S.T.D.’s, it’s seen as cleaner, sex is better, women like it. I predict
that a lot of men who can’t afford private clinics will start clamoring for it.”
(S.T.D.’s are sexually transmitted diseases.)
Male circumcision also benefits women. For example, a study of the medical
records of 300 Ugandan couples last year estimated that circumcised men infected
with H.I.V. were about 30 percent less likely to transmit it to their female
partners.
Earlier studies on Western men have shown that circumcision significantly
reduces the rate at which men infect women with the virus that causes cervical
cancer. A study published in 2002 in The New England Journal of Medicine found
that uncircumcised men were about three times as likely as circumcised ones with
a similar number of sexual partners to carry the human papillomavirus.
The suspected mechanism was the same — cells on the inside of the foreskin were
also more susceptible to that virus, which is not closely related to H.I.V.
Circumcision Halves H.I.V. Risk, U.S. Agency Finds, NYT, 14.12.2006,
http://www.nytimes.com/2006/12/14/health/14hiv.html?hp&ex=1166158800&en=299e031e4a678f86&ei=5094&partner=homepage
FDA panel to hear testimony on
antidepressants
Updated 12/13/2006 9:51 AM ET
From staff and wire reports
USA Today
WASHINGTON — Fierce debate is likely at a Food
and Drug Administration hearing Wednesday on whether antidepressants can prompt
adults to attempt suicide.
Opposing sides are lined up to speak before a
panel of scientific advisers to the FDA who are considering whether to recommend
expanding tough label warnings on such widely used drugs as Zoloft and Lexapro.
The agency ordered "black boxes," the most severe safety warning, on
antidepressants in 2004, saying the drugs increased suicidal behavior in
children and teens.
Treatment with antidepressants increases the risk of suicidal thoughts and
behavior in patients age 24 and younger, according to proposed changes to the
drugs' labels unveiled by federal health officials. The proposed changes would
expand a warning now on the labels that applies only to children and adolescents
treated with the drugs.
The Food and Drug Administration put forth its plan to update the drug labels
early Wednesday at the start of a meeting of outside advisers convened to
discuss the proposal. The changes also would include a recommendation that
patients of all ages be carefully monitored, especially when beginning
antidepressant treatment.
Adult antidepressant studies carried out by pharmaceutical companies suggest
that adults up to age 24 are more than twice as likely to think about or try
suicide if they're using antidepressants than if they're taking sugar pills. The
studies didn't find that antidepressants increase suicidal behavior for adults
ages 25 to 64, and the drugs appear to reduce suicide attempts in patients 65
and older.
The research is flawed, says psychiatrist Joseph Glenmullen, a clinical
instructor at Harvard Medical School, who is scheduled to testify Wednesday.
Most antidepressant studies exclude patients who are seriously suicidal, "but
suicidal people are the first to get these drugs, so how much can we know if
they're not even in the studies?" he says.
Also, the FDA summarizes the risk for patients 25 to 64 years old. Patients in
some subgroups are at heightened risk. For example, those 45 to 54 years old
have about the same higher risk as youths up to age 24, says Glenmullen, author
of Prozac Backlash and The Antidepressant Solution.
Another critic questions why the FDA told companies to consider suicidal
behavior as linked to the antidepressants only if it happens while a patient is
on the drug or within one day of either stopping or beginning to taper off
treatment.
"More than half of these drugs have very clear withdrawal symptoms that can be
dangerous and go on for weeks," says David Healy, a psychiatry professor at
Cardiff University in Wales.
Many psychiatrists and spokesmen for mental health advocacy groups do not want
the FDA to broaden black-box warnings.
"Untreated depression causes more loss of life than any of the approved
treatments," says Carolyn Robinowitz, president-elect of the American
Psychiatric Association. In her 38 years of practicing psychiatry, she says she
has never seen an increase in suicidal behavior, or a suicide, due to
antidepressants.
Black boxes have sharply reduced antidepressant use by kids, Robinowitz adds. "A
black box for adults creates a fear mentality, not only in patients but in
doctors. … It can limit access to needed care."
Any black box ordered by the FDA "should acknowledge clearly that untreated
depression is directly linked to suicide and that antidepressant medications are
effective in treatment," says Ken Duckworth, medical director of the National
Alliance on Mental Illness.
Contributing: Marilyn Elias, USA TODAY; Associated Press
FDA
panel to hear testimony on antidepressants, UT, 13.12.2006,
http://www.usatoday.com/news/health/2006-12-12-fda-antidepressants_x.htm
U.S. health system unprepared, report says
Posted 12/12/2006 11:21 PM ET
USA Today
By Mimi Hall
WASHINGTON — The nation's public health system
— the first line of defense against pandemic flu, a bioterror attack or other
widespread health emergency — remains woefully unprepared to protect the
American public, according to a report out Tuesday.
The annual study by Trust for America's Health
found that emergency health preparedness remains inadequate five years after the
9/11 and anthrax attacks of 2001 raised fears of bioterrorism. The study also
comes one year after Hurricane Katrina highlighted the need for the government
to provide health care quickly when thousands of people are in need.
"The nation is nowhere near as prepared as we should be for bioterrorism, bird
flu and other health disasters," said Jeff Levi, director of the trust. "We
continue to make progress each year, but it is limited. As a whole, Americans
face unnecessary and unacceptable levels of risk."
The report ranked the 50 states and Washington, D.C., on a 10-point system that
assessed key indicators, such as whether each state is capable of distributing
drugs and antidotes from a national stockpile, whether there are enough hospital
beds and nurses to handle a patient surge, and whether states have enough labs
and scientists to test for biological threats and other outbreaks.
Oklahoma came out on top — the only state that satisfied all 10 measures.
California, Iowa, Maryland and New Jersey scored lowest with four points out of
10.
The report also found that:
•The Centers for Disease Control and Prevention (CDC)has determined that only 15
states have been certified as capable of distributing drugs and antidotes from
the stockpile.
•Half the states would run out of available hospital beds within two weeks of a
moderate pandemic flu outbreak.
•Forty states face a nursing shortage.
•Rates for vaccinating the elderly for the flu decreased in 13 states over last
year.
•Four states don't test for the flu year-round, something health officials
regard as key to monitoring for a pandemic.
•Eleven states and Washington, D.C., aren't well-equipped to test for biological
threats.
Although Congress appropriated $5 billion this year for officials to prepare for
a possible pandemic, progress has been too slow, the report said.
"The public believes that more is being done and that we are better prepared
than we are," said trust board member Margaret Hamburg, a former New York City
health officer and former top official at the federal Department of Health and
Human Services. Allowing public health system weaknesses "to persist can lead to
serious consequences," she says. "Our systems need to be strengthened."
CDC spokesman Von Roebuck agreed. After decades of neglect, the public health
system has improved tremendously since 9/11, he said. But "more needs to be
done."
U.S.
health system unprepared, report says, UT, 12.12.2006,
http://www.usatoday.com/news/health/2006-12-12-us-system_x.htm
Connecticut Takes a Lead in Stem-Cell
Research Aid
December 10, 2006
The New York Times
By JENNIFER MEDINA
HARTFORD, Dec. 7 — One researcher will get
$3.5 million to explore how embryonic stem cells might be used to repair skin,
muscles, cartilage and bones badly injured in war. Another will get a few
hundred thousand dollars to examine ways such cells could repair neurons damaged
by epilepsy and seizures. A third will track their use in treating Parkinson’s
and other degenerative brain diseases.
Doling out $20 million to 21 research projects, Connecticut is moving faster and
further than other states to take the most controversial form of stem-cell
research, that involving tissue from human embryos, from the political arena to
the laboratory. The money will flow beginning next year, and is just a start:
the state has allocated $100 million over the next decade.
Connecticut leads several states that have started to take more aggressive steps
to support stem-cell research since President Bush vetoed a provision last
summer to spend federal money on it. That veto came after a White House move in
2001 to restrict federally funded research to a limited number of stem-cell
lines.
Soon California will begin to decide how to distribute nearly $150 million. New
Jersey has already awarded nearly $10 million, though most of the research
projects there do not involve embryonic cells; the state plans to allocate
millions more for additional research facilities. Maryland and Illinois have
also agreed to finance stem-cell research.
Private companies and foundations have also paid for some projects.
It will take years, if not decades, for the research proposals Connecticut is
supporting to be translated into clinical trials for therapies or cures. Each
proposal reflects the high expectations for the use of embryonic cells, which
scientists believe can be developed into any type of body tissue and thus
provide the basis for comprehensive treatments.
Opponents of such research, which uses destroyed embryos, want it to be severely
restricted or banned outright as inhumane.
The first round of grants will pay for research at the University of Connecticut
— which received $12 million, more than half the total — Yale University and
Wesleyan University.
“Certainly we put an emphasis on research that cannot or is not being done
elsewhere, which means embryonic stem cells,” said Dr. J. Robert Galvin, the
commissioner of Connecticut’s Department of Public Health and chairman of its
stem-cell advisory board. “We want to create an atmosphere here where we are
showing that this is welcome in Connecticut. We are operating under the belief
that if we build it, they will come.”
More than 70 proposals were considered by scientific review boards before being
submitted to the research advisory committee appointed by the state.
One rejected proposal included a plan to clone a human embryo and use it to
produce new stem cells. Dr. Galvin said that it was turned down because it
received low marks from the review board, and that there was no vocal opposition
to the idea of cloning embryos in principle.
The committee made a special effort to finance scientists who are only beginning
such research.
Dr. David W. Rowe, a professor of genetics and developmental biology at the
University of Connecticut Health Center in Farmington, who will get $3.5 million
for experiments on healing serious war wounds, said he was confident that such
work would bring attention to the university.
Like several other researchers receiving the grants, Dr. Rowe has done some
research using adult stem cells, but is hoping that embryonic cells will be
stronger and more efficient to repair the most serious wounds.
“What’s going on with soldiers now is that injuries are so catastrophic, massive
injuries and losing large segments of bone,” Dr. Rowe said. “This really is
transformative for us because we can look at ways of doing things we had only
thought about so far.”
Laura Grabel, a professor of biology at Wesleyan, whose $800,000 research
project focuses on epilepsy, has done some similar work using stem cells in
mice, and said that using human stem cells would take the research one step
closer to an ability to apply it in patients.
“We’re not anywhere near the level of this happening in humans, and there are
many roadblocks,” Dr. Grabel said. “But there are some models of the system that
look very promising, and we’re just at the very beginning. This is going to be
far beyond what we were able to do before.”
Like other scientists pushing for such research, Dr. Grabel said she was
thankful for the “extremely supportive political climate” in the state and said
she expected other researchers to take notice.
“There is no question that more money is going to translate into more research,
and we will be in an excellent position to get more dollars from the federal
government if the funds ever become available,” she said.
While the financing is widely praised in scientific circles and many advocates
view it as a potential boon for local economies, some caution that financial
support from states alone could result in a patchwork and haphazard approach to
the research.
“There are many states moving money around, but the implementation is not the
same everywhere, so while we are grateful for state governments filling the
void, it points to real problems,” said Sean Tipton, president of the Coalition
for the Advancement of Medical Research and a leading lobbyist in Washington for
stem-cell research funding.
“It is quite inefficient to have every state trying to figure out their own way
of how to review grants, spend the money and monitor what the researchers are
doing,” he said.
Connecticut Takes a Lead in Stem-Cell Research Aid, NYT, 10.12.2007,
http://www.nytimes.com/2006/12/10/nyregion/10stem.html
Troubled Children
Off to College Alone, Shadowed by Mental
Illness
December 8, 2006
The New York Times
By LYNETTE CLEMETSON
Her mother called it a negotiable proposition.
But to Jean Lynch-Thomason, a 17-year-old with bipolar disorder who started
college this fall, her mom’s notion to fly from their home in Nashville to her
campus in Olympia, Wash., every few weeks to monitor Jean’s illness felt
needlessly intrusive.
“I am so totally aware of the control you have over me right now,” Jean said,
sitting in her parents’ living room one evening last June, before coolly
reminding her mother of her upcoming 18th birthday. “In a few months the power
dynamic is going to be different.”
For Chris Ference, 19, who is also bipolar, the fast-approaching autonomy of his
freshman year held somewhat less appeal. His parents had always directed every
aspect of his mental health care. Last summer, over Friday night pizza at his
home in Cranberry Township, Pa., he told them that assuming control felt more
daunting than liberating.
“If it was up to me, I would just have it so you could make those decisions for
me up until I was like, 22,” he said. “I mean, you’ve raised me well up to now.
You know me better than anyone.”
The transition from high school to college, from adolescence to legal adulthood,
can be tricky for any teenager, but for the increasing number of young people
who arrive on campus with diagnoses of serious mental disorders — and for their
parents — the passage can be particularly fraught.
Standard struggles with class schedules, roommates, and sexual and social
freedom are complicated by decisions about if or when to use campus counseling
services, whether or not to take medication and whether to disclose an illness
to friends or professors.
Keeping a psychiatric disorder under control in an environment often fueled by
all-night cram sessions, junk food and heavy drinking is a challenge for even
the most motivated students. In addition, the normal separation that goes along
with college requires new roles and boundaries with parents, the people who best
know the history and contours of their illness.
Like Jean and Chris, young adults approach the move to a new life differently,
some with defiant independence, some with avoidance. Each approach, say
psychiatrists, counselors, dormitory assistants and other campus leaders, comes
with its own risk. The students who are most dependent on their parents may be
dangerously unprepared for the inevitable stresses of college life. On the other
hand, students who are adamant about doing everything on their own may be afraid
to reach out for help when they stumble.
For parents, the anxious pride at seeing children go off to college is often
tinged with fear that their child might fall apart, spiraling into depression or
becoming suicidal. Are they going to therapy as they promised? Are they taking
the right dose of medication at the right time? Should they as parents inform
the school that their child has an illness? Is a fight with a roommate part of a
normal transition to college life or a sign of impending trouble? Does an
emotional e-mail message written at 3 a.m. represent a transitory moment of
turmoil or a reason to get on an airplane?
Once teenagers legally become adults, which in most states happens at age 18,
they, not their parents, assume control over decisions about therapy and
medication. If trouble arises, parents may or may not hear about it because
college counselors are bound by confidentiality when dealing with adult
students.
The Trauma of Separation
For Jean, as for many teenagers coping with mental disorders, just getting
through high school was an ordeal. After experimenting with home schooling, a
high pressure prep school and an outdoor learning academy geared to nature
activities, Jean, a bright student with inconsistent grades but high SAT scores,
decided to forgo her senior year and find a college that would take her without
a high school diploma.
She was accepted at Evergreen State College in Olympia, Wash., a nontraditional
college of roughly 4,400 students that issues written evaluations in place of
letter grades.
Evergreen’s environmental focus — the campus has its own organic farm,
composting program and a contest for commuters who bike, walk or carpool to
campus — felt like a good fit for Jean, who is passionately committed to the
environment and social justice.
A consciously quirky teenager who sews her own clothes (to avoid crass
consumerism, she says) and who prefers bus trips to flying (to avoid
contributing to the pollution caused by air travel), Jean is disarmingly
straightforward and self-aware.
She said she stopped taking medications when she was 14 because the side effects
left her feeling “out of whack and emotionally inauthentic.”
She is determined to stay off medications during college, and she devoted
considerable advance thought to possible triggers for her illness, like the long
rainy winters of the Pacific Northwest.
“I don’t feel vulnerable about this transition because this is very much my
decision,” she said. “This is a very autonomous move, very much me structuring
my own life. I feel like I am putting myself in a situation with really clear
intentions.”
Jean’s parents, Amy Lynch, 52, and Phil Thomason, 53, were hesitant when Jean,
the younger of their two daughters, refused to take medications after eighth
grade. Her childhood and early adolescence had been a whirlwind of depression,
rage and experiments with different medications and treatments.
But when Jean was about 14, Ms. Lynch and Mr. Thomason said, she began to seem
more stable. Her developing coping skills, combined with reports about negative
side effects of psychotropic drugs in children, persuaded them to acquiesce to
her demands to ride out the swings of her illness drug free.
They said they believed Evergreen would be a good college for Jean. Still, the
move — to someplace so far from home — made them anxious. In the months before
Jean left, Ms. Lynch said she wanted her to go back on medication to smooth the
adjustment to college life, a suggestion that Jean adamantly rejected.
Ms. Lynch worried that Jean took for granted the tacit stability of being at
home.
When Jean’s depression sets in, she tends to close herself off from people. At
home, Ms. Lynch said, “I can look at Jean and know in five minutes what’s going
on with her and how to respond to it.”
At such a distance it will be difficult to catch the signs.
“I feel like we’re doing a high-wire act,” she said, “and I am not sure we have
a strong enough net.”
Rummaging through the accumulated possessions of adolescence in her bedroom over
the summer, Jean singled out the items that she could not leave without: her
sewing machine, her coffee maker, the social justice posters that covered her
wall.
With her mother out of earshot, she acknowledged that she understood her
parents’ angst. “I get that this is intense for everyone,” she said. “I do.”
Hesitant to Leave the Nest
The uncertain months between high school and college were also anxious ones for
Chris Ference and his parents.
Still groggy from an early morning drive to campus, his husky 6-foot-2 frame
jammed into an auditorium chair in the student union, Chris shifted
uncomfortably as a freshman orientation coordinator welcomed new students and
their parents to the Behrend College, a Pennsylvania State University satellite
campus in Erie, Pa.
“Today really is the first day of your freshman year of college,” the cheery
administrator told the group on a June morning more than two months before the
start of fall term.
Chris had initially been reluctant to go away to college. Though eager to leave
the rigid structure and peer pressure of high school, where he told few friends
about his illness, he preferred the idea of living at home during college and
commuting to an engineering program in nearby Pittsburgh.
It was his mother, Debbie Ference, a service director with the southwestern
Pennsylvania division of the National Alliance on Mental Illness, an advocacy
group, who nudged him to move away.
He chose Behrend for its strong engineering program and small student body of
about 3,700.
A boyish and fidgety teenager who likes heavy metal music, Xbox games and
anything having to do with electronics, Chris said he had given little advance
thought to his new responsibilities in college.
Just days before his orientation, he listened passively as his father, Michael
Ference, and Ms. Ference talked about his care at school. They wondered aloud
about whether he would be able to continue seeing his longtime therapist in
Pittsburgh, more than two hours away. They raised the possibility of putting an
advance mental health directive in place, so that they could be contacted if
Chris was ever in crisis and unable to consent to parental notification.
They discussed how they worried about the possibility of Chris mixing alcohol
with his medications. Chris huffed in annoyance and told them he was “smart and
moral enough” not to fall into that trap.
The fact that Chris was willing to engage in the discussion at all was a sign,
they said, of progress.
Chris was first hospitalized and received a diagnosis of bipolar disorder at age
10 after a severe episode of depression, mania and suicidal thoughts. He was
hospitalized again briefly in sixth grade, after the lithium that had stabilized
him for two years became ineffective.
But successful therapy and medication since then have kept the illness at a
manageable level. He graduated from high school with honors, and in his senior
year saw his therapist only every six weeks. A recent medication adjustment has
left him able to feel and express more than he has in years.
“This whole move is like a coming-out process,” said Mr. Ference, 50, a service
coordinator for families with autistic children. “Up to now it’s been all
parental motivation. But I think this is a healing process for him after so many
hard years.”
In a 2005 national survey of the directors of college counseling centers, 95
percent of counseling directors reported an increase in students who were
already on psychiatric medications when they came in for help. While
universities grapple with how to serve the growing number of students with
mental disorders, students are taking the initiative by helping one another.
Active Minds, a student-led mental health advocacy organization founded in 2001
at the University of Pennsylvania, now has 56 chapters at schools including
Georgetown University, Columbia University, the University of South Florida and
the University of Maryland.
The National Alliance on Mental Illness has 30 campus affiliates, with 18 more
in formation, groups that are set up as student clubs and are financed by school
activity budgets and fund-raisers. Programs like the Jed Foundation, a suicide
prevention program, and National Depression Screening Day, held each October,
offer additional resources.
While the overall message from the groups and programs focuses on the potential
for success, students who have been through the transition of leaving home for
college say it is also important to be honest about the challenges.
Difficult Experiences
Stacy Hollingsworth, an honors student at Rutgers University who suffers from
major depressive disorder, dropped out of college in the fall semester of her
sophomore year after the routine aspects of college life left her so
incapacitated that she became suicidal and was hospitalized.
At home in Old Bridge, N.J., she could retreat to the isolation of her bedroom
when she was depressed — an impossibility in her crowded dormitory. The
staggered class schedule left her lacking a dependable rhythm. Even getting
dressed and walking to the cafeteria became an insurmountable task.
“I was in excruciating pain. I couldn’t breathe,” she said.
Though she had been suffering from depression since her early teens, she hid her
struggle from family and friends. She sought counseling help for the first time
in college, but still could not cope.
After a two-year absence and the loss of $15,000 in state scholarships, Ms.
Hollingsworth, now 22, is back at Rutgers finishing her degree in exercise
physiology and psychology. She is founder of the Rutgers’ affiliate of the
National Alliance on Mental Illness, one of the organization’s newest student
chapters.
At 37, Robert C. Haggard III, who three years ago founded a chapter of the same
organization at Washburn University in Topeka, Kan., is still working on his
bachelor’s degree in studio art.
During his first attempt at college, right out of high school, Mr. Haggard said,
“I wasn’t honest with myself that I needed assistance.”
He tried to blunt the increasing severity of his bipolar disorder with alcohol,
a common tactic for students with psychological disorders, experts say.
He was on academic probation when, in 1992, he withdrew from school. He
struggled though several jobs, a variety of medications, and a suicide attempt
at age 29 before he started to get his condition under control.
It has only been within the past four years, he said, that he has gained
stability. “I study during the day, sleep at night, eat right and maintain a lot
of structure and routine,” he said. “It sounds simple, but it can be a hard
place to get to.”
Dr. Richard Kadison, chief of mental health services at Harvard, said there were
things students with mental illness could do before starting college to increase
the chances of a manageable transition.
Most important, he said, is establishing local health support on or near campus.
Maintaining a relationship with a counselor from home can be helpful, but “you
don’t want to end up in an emergency talking to someone at school that you have
never laid eyes on,” Dr. Kadison said.
Last-Minute Worries
After the opening session of freshman orientation at Behrend College back in
June, Chris Ference disappeared into a pack of students to begin selecting his
classes.
His mother headed in the opposite direction and wandered into a session on
student support networks led by Sue Daley, the director of the counseling
office. She listened intently as the counselor talked about problems students
had encountered in recent years.
She winced when the counselor related the story of a young woman who had a
psychotic episode the previous year, during which she ripped tiles from her
dormitory room ceiling because she believed the F.B.I. was monitoring her.
“We sent her home so she could get her emotional self together,” Ms. Daley told
the group.
After the session, Ms. Ference complained that it sounded as if the goal of the
counseling center was to get the “crazy kids” out of the way.
“I was offended by that,” she said to Ms. Daley. “I want to be comfortable
enough with this school that I know you will take care of my son.”
In the car on the way home from the campus visit, Ms. Ference mentioned her
discomfort with the counseling presentation.
“We definitely have to put some outside counseling support in place, just in
case you don’t like it there,” she said to her son.
Looking through his thick pamphlet of brochures from the day, Chris responded,
“Hey, we get a discount on computers and iPods!”
Ms. Ference took a hand off the steering wheel to rub at the stress headache
pulsating at her temple.
About the same time in June at Bongo Java, a trendy coffee shop near her home in
the Belmont-Hillsboro section of Nashville, Jean Lynch-Thomason pulled out a
tattered journal, held together with silver duct tape. A picture of herself in
the third grade, taped to the cover of the thick diary, stared back at her as
she gathered her thoughts.
As she prepared for college, she had been writing in the journal several times a
day.
More pensive than during the previous meeting when she matched wits with her
parents about her desired independence, Jean confessed that she had been
thinking quite a lot about her move in the fall.
“There is a lot more fear and anxiety about this transition than I am letting
on,” she said. “We can set up all the protective measures we want and still
there is just no way to tell what is going to happen, and man, that’s hard.”
She remained determined not to let her mother fly out to Washington to check on
her. And she resolved to limit her own trips home, to cut down on unnecessary
air travel.
But she said she felt confident that she had done the most optimal planning
possible. She had decided to have an apartment by herself so that she could
prepare her own vegan meals. Living alone, she said, would also afford her the
privacy to sleep well and have the solitude she craves when her depression sets
in.
That solitude, she added, might be a double-edged sword in a new environment
where she would be more reluctant to engage with people during dark periods of
depression.
“I am in a good, copacetic place right now,” she said. “But I also know that
there is every possibility that things could go bad. I just sort of feel like if
I get out there and don’t do well, then I am letting everyone down.”
Back at home soon after, she breezed past her mother, confident as ever.
A New Perspective
Three months after arriving on campus, Jean’s anger at her parents’ concern
seems to have receded. Her mother’s hotly debated first visit came and went in
October. There were no confrontations over medication, no accusations of
heavy-handedness.
Mother and daughter said little at all, in fact, about the illness that has so
defined their lives, and their relationship, choosing instead to ride bikes,
work at a free store for the needy, and play in a fountain one night in the
center of downtown.
“I’m more settled, I guess,” said Jean, who will turn 18 next week. She was
surprised that she so enjoyed the visit. “I was in a good place. She was in a
good place. My illness just didn’t particularly seem relevant.”
Some ideas that had made sense in the abstract — like living alone — felt unwise
after she arrived in September and looked at a few apartments. When a friend
from Tennessee offered her a tiny crawl space of a room in an overcrowded home
he shared with several other students off campus, Jean said it felt just right.
“It’s not like I’m going up to people saying, ‘Hi, I’m Jean, I’m bipolar,’ ” she
joked. “But I’m surrounded by beautiful supportive people, and I know if I need
it, they will call me out.”
She has maintained sessions by telephone with her therapist back home every two
weeks. But she has also met people at the campus counseling center. She said she
liked that they encouraged holistic as well as purely medical approaches to
treatment, and that she would not hesitate to seek help there if the need arose.
Back in Nashville, Ms. Lynch said she may have underestimated her daughter’s
ability to make good decisions for herself. The lushness and environmental
consciousness of Evergreen and the surrounding area seemed to have a stabilizing
effect on Jean, she said. There was not a trace of the early signs of mania or
depression that Ms. Lynch could usually spot in her daughter well before others.
She said she had decided not to raise the issue of medication again. For now. “I
may have a different answer a few months from now,” she said. “But what I know
today is that she seems to have learned a lot about coping. And that’s how we
get through this, by what we know any given day.”
Chris Ference has also changed since he packed his things and left home in late
August. Sitting on the bed in his dorm room, sounding more mature than he had a
few months earlier, he said the transition was smoother than he had anticipated.
But he was still working out some of the particulars of dealing with his bipolar
disorder. He told his roommate about his illness in mid-October, only because a
reporter was coming to their room for an interview.
“It’s cool. He’s cool. It’s fine,” he said, with a hint of wariness. “It’s
probably good for him to know anyway, so he can understand it, in case I ever
need him to help me out.”
Discreetly taking his medications in a dorm room typically crammed with
engineering students until the wee hours of the morning is also a challenge. In
an effort not to draw attention to himself, he said, he takes his two
medications late at night, right before he lays his head down to sleep. If
anyone notices, they have not let on.
He and his mother met with Ms. Daley, head of the counseling center, before
school started. After the unpleasant encounter at summer orientation, Ms.
Ference wanted some assurances that the school’s services were adequate. She
left satisfied, she said, and Chris seemed comfortable enough with the
counseling center to go there if he needed to.
Chris said he doubted he would need help from Ms. Daley or anyone else at the
center. He has friends and is playing guitar in a band, he keeps his partying
“under control,” and he loves his engineering classes.
He is under no illusions about his illness, he said. He knows it will be
something that he has to learn to manage throughout his adult life.
“But things are just going so good,” he said. “So far.”
Off
to College Alone, Shadowed by Mental Illness, NYT, 8.12.2006,
http://www.nytimes.com/2006/12/08/health/08Kids.html?hp&ex=1165640400&en=6d958028c9812fe6&ei=5094&partner=homepage
Minnesota sets bar on health again
Updated 12/5/2006 7:36 AM ET
USA Today
By Liz Szabo
Americans looking for a long life may want to
move to Minnesota.
The land of 10,000 lakes has been named the
healthiest state for the fourth consecutive year, according to a report released
today by the United Health Foundation, a private, non-profit organization
dedicated to supporting healthy communities.
The foundation, which worked with American
Public Health Association and Partnership for Prevention, rated each state on 20
key measures of wellness, such as the rates of cancer, smoking, car accidents
and high school graduation.
Minnesota, whose score is 21% higher than the national average, stands out for
many reasons, the report says. It has the country's lowest uninsured rate, 8.4%,
and few children live in poverty or die as infants.
Gov. Tim Pawlenty credits a state health insurance program for the working poor,
along with social safety-net programs for children, for helping Minnesotans get
the medical care they need.
The state also has an outdoorsy, health-conscious culture and a low unemployment
rate, Pawlenty says.
Louisiana, whose uninsured population rose 9% in the past year, ranked as the
unhealthiest state. Louisiana has fared near the bottom since the rankings began
in 1990. The state faces many challenges confronting the rest of the USA: high
rates of obesity, children in poverty, infant mortality and overall premature
death.
Overall, the nation's health is stagnating, says Reed Tuckson, the foundation's
senior vice president. Though health has improved by nearly 19% since 1990,
researchers say, nearly all of that progress was made during the '90s. The
country is no healthier today than it was in 2000, Tuckson says.
Several key problems, such as obesity, tobacco use and the large number of
uninsured, are damaging the nation's health. In 1990, 11.6% of Americans were
obese. Today, nearly 25% are obese.
The number of Americans who are uninsured has grown from 13.4% to 15.9% in the
same time, according to the report.
The infant mortality rate is especially shameful, Tuckson says. In a list of 36
nations, the USA shares last place with the tiny countries of Andorra, Cuba,
Croatia and Estonia.
The nation also could be much healthier if fewer people smoked, says Corinne
Husten of the Centers for Disease Control and Prevention. Fewer than 7% of women
in the U.S. Virgin Islands smoke, Husten says, suggesting it's possible to
reduce the nation's overall adult smoking rate of 21%.
Smoking causes about 440,000 deaths a year in the USA, the American Heart
Association says.
Even at No. 1, Pawlenty says, Minnesota can still do better.
"We are not living as healthy as we should, and we are dying too soon. There is
nothing more worthy of a sustained national conversation than this."
Minnesota sets bar on health again, UT, 5.12.2006,
http://www.usatoday.com/news/health/2006-12-05-minnesota-health_x.htm
Ads target stigma of mental illness among
youth
Updated 12/4/2006 12:32 AM ET
By Donna Leinwand
USA Today
WASHINGTON — The federal government is
launching a $1 million public service campaign beginning today aimed at reducing
the stigma surrounding mental illnesses such as depression and bipolar disorder.
Health agencies say millions of U.S. adults go
untreated for mental illnesses because they are too ashamed to tell friends and
family. The government's campaign will use public service radio and TV ads to
encourage young adults to stand by their peers. Later phases of the campaign
will address older people and rural areas.
"If you have early and consistent support from your peers and you get
appropriate treatment, then you have a much better chance of managing the
illness over time," says Kathryn Power, director of the Center for Mental Health
Services of the U.S. Substance Abuse and Mental Health Services Administration
(SAMHSA) in Rockville, Md.
"It's important as a friend in a relationship with someone recovering from
mental illness that you exhibit social acceptance."
When Cara Anthieny, 18, spent a week in the hospital dealing with depression,
her school friends, bearing books, flowers and candy, traveled more than 100
miles to visit with her. She says their support speeded her recovery.
"They really understood. I wasn't embarrassed," says Anthieny, a student in
Chico, Calif. "It was important to me not to feel like an outcast."
But SAMHSA and the Center for Mental Health Services say millions of other
adults go untreated because they are too ashamed to tell friends and family.
In 2005, nearly 25 million people 18 or older had some type of serious
psychological distress — about 11.3% of the population overall and 18.6% of
young adults ages 18 to 24, according to the National Survey on Drug Use and
Health. Of the 13.5 million people who did not seek treatment, 26% cited as one
of their primary reasons the stigma associated with mental illness.
"We're trying to change the social norm," says Peggy Conlon, president and CEO
of Ad Council, an industry-supported group that creates public service messages.
The council decided to target friends instead of the patients themselves after
focus groups and surveys showed that young adults were not educated about
treatment for mental illness, she said.
"We felt that engaging friends would not only encourage people to get help, but
it would also destigmatize the issue," she said.
"We wanted to help them understand that people can recover and there's a role
for them as friends to play."
Ads
target stigma of mental illness among youth, UT, 4.12.2006,
http://www.usatoday.com/news/health/2006-12-03-mental-ad_x.htm
Sen. Obama joins evangelicals in AIDS fight
Fri Dec 1, 2006 10:51 PM ET
Reuters
By Jill Serjeant
LAKE FOREST, California (Reuters) - Democratic Sen. Barack
Obama and a leading U.S. evangelical pastor pledged on Friday to work together
in an unusual and controversial meeting of minds on the fight against AIDS.
Obama, an abortion rights supporter from Illinois and a potential presidential
contender, and Rick Warren, leader of the Saddleback Valley Community Church,
brushed aside criticism from some conservative church leaders angry at Obama's
presence at an AIDS conference designed to rally Christians to fight the global
pandemic.
"It is time for a coalition of civility. We can treat each other with respect
and work together," said Warren, joining hands and standing in prayer with Obama
and Republican Sen. Sam Brownback of Kansas, another conference speaker and
possible presidential contender.
"Our goal has been to put people together who normally won't even speak to each
other," said Warren, author of a best-selling inspirational book, "The Purpose
Driven Life."
Eighteen leaders in the anti-abortion movement sent Warren a letter this week
saying Obama had no place at the Saddleback pulpit because of his stance on
abortion.
"If Sen. Obama cannot defend the most helpless citizens in this country, he has
nothing to say to the AIDS crisis. You cannot fight one evil while justifying
another," said the letter signed by the presidents of the American Family
Association, the American Life League and others.
Obama told the conference of some 2,000 Christians, AIDS organizations and
church leaders from 18 countries that AIDS required a "change in hearts and
minds, in cultures and attitudes."
"AIDS is a challenge not only of our willingness to respond but of our ability
to look past the artificial divisions and debates that have often shaped that
response," said Obama, winning a standing ovation from the audience.
'LATE TO THE PARTY'
The conference was organized on World AIDS Day by Warren's church in Southern
California, which attracts some 22,000 people to services each week and is one
of the largest churches in the United States.
Warren said evangelicals -- some of whom have regarded AIDS as God's punishment
for gay sex -- had been "late to the party in this particular crisis."
"We have had to repent over that. But now we are here to stay," said Warren. "It
is the church that needs to take the lead on HIV/AIDS."
Obama said he respectfully disagreed with people who oppose condom use as a
means of HIV/AIDS prevention because they believe it encourages promiscuity.
"I do not accept the notion that those who make mistakes in their lives should
be given an effective death sentence," he said.
The Saddleback church says the aim of the conference was to encourage millions
of Christians in the United States and around the world to become caregivers,
use churches as centers for help and campaign to prevent AIDS at home and in
Africa.
In a bid to reduce the stigma of AIDS testing, Obama, Warren and Brownback each
took a mouth swab test for the disease during a news conference. The results,
back in 20 minutes, were negative for all three.
Sen. Obama joins
evangelicals in AIDS fight, R, 1.12.2006,
http://today.reuters.com/news/articlenews.aspx?type=domesticNews&storyID=2006-12-02T035038Z_01_N01387138_RTRUKOC_0_US-AIDS-EVANGELICALS.xml&WTmodLoc=Home-C5-domesticNews-3
Medicaid Plan Prods Patients Toward Health
December 1, 2006
The New York Times
By ERIK ECKHOLM
HAMLIN, W.Va. — No question, John Johnson is a
doctor’s nightmare.
Speaking from the easy chair where he spends his days in a small wooden house
near this small Appalachian town, his left trouser leg folded by a safety pin
where a limb was lost to diabetes, he lighted another cigarette.
Mr. Johnson, 61 and a former garbage collector, takes insulin and goes to a
clinic once a month for diabetes checkups. Taxpayers foot the bill through
Medicaid, the federal-state health coverage program for the poor.
But when doctors urged him to mind his diet, “I told them I eat what I want to
eat and the hell with them.”
“I’ve been smoking for 50 years — why should I stop now?” he added for good
measure. “This is supposed to be a free world.”
Ignoring doctors’ orders may now start exacting a new price among West
Virginia’s Medicaid recipients. Under a reorganized schedule of aid, the state,
hoping for savings over time, plans to reward “responsible” patients with
significant extra benefits or — as critics describe it — punish those who do not
join weight-loss or antismoking programs, or who miss too many appointments, by
denying important services.
The incentive effort, the first of its kind, received quick approval last summer
from the Bush administration, which is encouraging states to experiment with
“personal responsibility” as a chief principle of their Medicaid programs. Idaho
and Kentucky are also planning reward programs, though more modest ones, for
healthful behavior.
In a pilot phase starting in three rural counties over the next few months, many
West Virginia Medicaid patients will be asked to sign a pledge “to do my best to
stay healthy,” to attend “health improvement programs as directed,” to have
routine checkups and screenings, to keep appointments, to take medicine as
prescribed and to go to emergency rooms only for real emergencies.
“We always talk about Medicaid members’ rights, but rarely about their
responsibilities,” said Nancy Atkins, state commissioner of medical services.
“We’re in an Appalachian culture where there’s a fatalism, and many people don’t
go in for checkups or preventive services,” Ms. Atkins said, noting that West
Virginia had some of the country’s highest rates of obesity, smoking, heart
disease and diabetes. “We want to reach people before they get chronic and
debilitating diseases that will keep them on Medicaid for the rest of their
lives.”
Those signing and abiding by the agreement (or their children, who account for a
majority of Medicaid patients here) will receive “enhanced benefits” including
mental health counseling, long-term diabetes management and cardiac
rehabilitation, and prescription drugs and home health visits as needed, as well
as antismoking and antiobesity classes. Those who do not sign will get federally
required basic services but be limited to four prescriptions a month, for
example, and will not receive the other enhanced benefits.
In future years, those who comply fully will get further benefits (“like a
Marriott rewards plan,” Ms. Atkins said), their nature to be determined but
perhaps including orthodontics or other dental services.
No one questions that West Virginia, more than most other states, needs more
healthful lifestyles and better primary and preventive care. But the new plan
has stirred national debate about its fairness and medical ethics. A stinging
editorial in The New England Journal of Medicine on Aug. 24 said it could punish
patients for factors beyond their control, like lack of transportation; would
penalize children for errors of their parents; would hold Medicaid patients to
standards of compliance that are often not met by middle-class people; and would
put doctors in untenable positions as enforcers.
“What if everyone at a major corporation were told they would lose benefits if
they didn’t lose weight or drink less?” said a co-author of the editorial, Dr.
Gene Bishop, a physician at Pennsylvania Hospital in Philadelphia.
Denying mental health aid to those who do not sign seems especially
counterproductive, Dr. Bishop said in an interview.
“If you think about the people least able to do simple things like keep
appointments and take all their medications,” she said, “people with mental
health and substance abuse problems are right up there.”
Judith Solomon of the private Center on Budget and Policy Priorities, in
Washington, said that the plan was unlikely to save West Virginia money or
improve patient health and that it carried “the risk that some very vulnerable
people may be denied health care they need.”
But Ms. Atkins, the state health official, said critics had misunderstood the
plan, which, she said, simply “gives people rewards and incentives to improve
their health.”
Here in Lincoln County, as in the two other counties in the pilot phase, there
is a federally subsidized primary-care center that is a leader in developing
“care management” programs, nudging people into preventive services and
lifestyle changes voluntarily.
The Lincoln Primary Care Center in Hamlin, a town of 1,500 an hour’s winding
drive west of Charleston, is a showcase for preventive medicine, with its own
fitness center, an exercise physiologist, a dietary adviser and a mental health
counselor — resources that are lacking in many rural clinics. The center stays
open until 9 most nights, making it easier for sick people to come in for urgent
care rather than driving to distant emergency rooms.
A former tobacco-growing area, Lincoln is one of the state’s poorest counties,
with a population of 22,000 scattered through the hills. About 9,000 use this
care center, a majority of them uninsured or on Medicaid, said Brian Crist, the
chief executive.
Some doctors here and throughout the state were initially alarmed by the new
rules, which were delayed six months for discussions and fine-tuning. But state
officials appear to have allayed some fears, and many doctors are now taking a
wait-and-see attitude.
Officials have offered assurances, for example — and Ms. Atkins emphasized in an
interview — that doctors will be able to provide medically necessary drugs and
care to children even if their parents have not followed the agreement. This was
not clear in the written plan and may be needed in any case, critics said, to
comply with federal law.
Dr. Syem B. Stoll, a physician at Lincoln, said the clinic’s three-year-old
effort to promote lifestyle changes for patients with hypertension, obesity,
diabetes and other problems had already made a difference for many.
“We’re doing a lot more than just giving people pills and sending them home,”
Dr. Stoll said.
Of the new Medicaid rules, he said: “My interactions with patients won’t change
— I am who I am. But giving people responsibility and initiative is the way to
go.”
In interviews with several residents of the Hamlin area, including Medicaid
recipients, none said they had heard about the new rules.
When they were outlined for Mr. Johnson, the cantankerous diabetic, he said he
had no intention of participating. “Hell, no,” he said. “I wouldn’t sign an
agreement like that.” Somewhat incongruously, he appears to be off the hook: as
a disabled person he will be exempt under the rules.
Brittney Lovejoy, 18, earns $5.40 an hour at the Burger King here and is a
Medicaid patient at the Lincoln center, as are her 4-year-old daughter and
6-month-old son. “I guess I’ll have to sign it,” she said after hearing a
description of the new agreement, apparently unenthusiastic about the idea
though not foreseeing any major problems.
But Karen Ball, 35, a night manager at the Burger King, said she did not think
the program was fair. Though Ms. Ball is uninsured herself — she uses the
Lincoln clinic rarely, paying reduced fees — her three sons are on Medicaid.
They go for their required annual checkups, so the agreement should pose no
problem for them.
Still, “some people can’t afford the transportation to go to these programs,”
she said, “between the price of gas and the lack of jobs here — and what jobs
there are pay minimum wage.”
Medicaid Plan Prods Patients Toward Health, NYT, 1.12.2006,
http://www.nytimes.com/2006/12/01/us/01medicaid.html?hp&ex=1165035600&en=f85476122a478646&ei=5094&partner=homepage
Editorial
When Don’t Smoke Means Do
November 27, 2006
The New York Times
Philip Morris has adopted the role of good
citizen these days. Its Web site brims with information on the dangers of
smoking, and it has mounted a campaign of television spots that urge parents, oh
so earnestly, to warn their children against smoking. That follows an earlier
$100 million campaign warning young people to “Think. Don’t Smoke,” analogous to
the “just say no” admonitions against drugs.
All this seems to fly against the economic interests of the company, which
presumably depends on a continuing crop of new smokers to replace those who drop
out or die from their habit. But in practice, it turns out, these industry-run
campaigns are notably ineffective and possibly even a sham. New research shows
that the ads aimed at youths had no discernible effect in discouraging smoking
and that the ads currently aimed at parents may be counterproductive.
That disturbing insight comes from a study just published in The American
Journal of Public Health by respected academic researchers who were supported by
the National Cancer Institute, the National Institute on Drug Abuse and the
Robert Wood Johnson Foundation. Using sophisticated analytical techniques, the
researchers concluded that the ads aimed directly at young people had no
beneficial effect, while those aimed at parents were actually harmful to young
people apt to see them, especially older teenagers. The greater the teenagers’
potential exposure to the ads, the stronger their intention to smoke and the
greater their likelihood of having smoked in the past 30 days.
Just why the costly advertising campaigns produce no health benefits is a rich
subject for exploration. The ads are fuzzy-warm, which could actually generate
favorable feelings for the tobacco industry and, by extension, its products. And
their theme — that adults should tell young people not to smoke mostly because
they are young people — is exactly the sort of message that would make many
teenagers feel like lighting up. (Trial testimony has made it clear that the
goal of Philip Morris’s youth smoking prevention programs is to delay smoking
until adulthood, not to discourage it for a lifetime.)
The most exhaustive judicial analysis of the industry’s tactics, by Judge Gladys
Kessler of the Federal District Court for the District of Columbia, concluded
that the youth smoking prevention programs were not really designed to
effectively prevent youth smoking but rather to head off a government crackdown.
They are minimally financed compared with the vast sums spent on cigarette
marketing and promotion; they are understaffed and run by people with no
expertise; and they ignore the strategies that have proved effective in
preventing adolescent smoking. The television ads, for example, do not stress
the deadly and addictive impacts of smoking, an emphasis that has been shown to
work in other antitobacco campaigns.
Philip Morris says it has spent more than $1 billion on its youth smoking
prevention programs since 1998 and that it devised its current advertising
campaign on the advice of experts who deem parental influence extremely
important. But the company has done only the skimpiest research on how the
campaign is working. It cites June 2006 data indicating that 37 percent of
parents with children age 10 to 17 were both aware of its ads and spoke to their
children about not smoking. How the children reacted has not been explored. And
somehow the company forgot to tell the parents, as role models, to stop smoking
themselves.
Philip Morris, the industry’s biggest and most influential company, is renowned
for its marketing savvy. If it really wanted to prevent youth smoking — and cut
off new recruits to its death-dealing products — it could surely mount a more
effective campaign to do so.
When
Don’t Smoke Means Do, NYT, 27.11.2006,
http://www.nytimes.com/2006/11/27/opinion/27mon1.html
Spending
For the Autistic, a Gift of Common Ground
November 26, 2006
The New York Times
By JULIE BICK
ALMOST everyone knows of a family affected by
autism, the disorder that can impair a child’s ability to form social and
emotional connections. The Centers for Disease Control and Prevention says
autism-related disorders are more common than cerebral palsy, Down syndrome,
blindness and deafness — some of the other major childhood disabilities.
Children with autism often have a hard time interpreting emotions in others, may
learn to speak later and can experience hypersensitivity to noise, light and
touch. As the holidays approach, family members and friends may wonder what to
buy for any children with autism on their list while accommodating their special
needs.
Play is the work of all children because it lets them practice new skills, find
new interests and develop mentally and physically. For a child with autism, the
right kind of play at the right time is crucial. Play can help deliver some
basic communication and life skills that may not come naturally.
Children with auditory processing problems, for example, may have trouble
translating a verbal request like “put on your coat” into a physical action.
Games like the Hullabaloo DVD Game ($24.99), from Cranium, which playfully asks
children to jump to different floor pads while showing others performing the
desired action on screen, combine auditory and visual cues that can help
children follow directions.
Although some toys are specially designed for children with autism, many
families may prefer toys from the mainstream, like the Hullabaloo game. Lauri
Perry of Seattle, the mother of Clark, an 8-year-old with autism, buys
off-the-shelf items rather than custom products. “I want him to have the toys
everyone else has, so when kids come over, they see things they know and like,”
she said. “His room shouldn’t look like a therapy station.”
On the other hand, a specially designed therapeutic toy can focus more directly
on a specific skill, like maintaining appropriate eye contact.
Autism disorders fall on a spectrum from mild to very severe. Mari Stobbe, a
founder of the Autism Spectrum Treatment and Research Center of Seattle, said:
“It is vitally important to help children on the spectrum develop social
connections, and to find common ground with typically developing children.” She
said a shared interest in a toy could help foster that bond.
Ms. Perry also wants to make sure Clark has popular items. One favorite is a
SpongeBob SquarePants backpack with a cloth tongue that rolls out when he unzips
it. “The other kids think it’s cool,” she said.
For the past 13 years, Toys “R” Us has published a “Toy Guide for
Differently-Abled Kids,” originally in print and now at
www.toysrus.com/differentlyabled. It offers tips for buying mainstream toys
based on factors like multisensory appeal, safety and potential for interaction.
The toys in the catalog have been evaluated by Lekotek (www.lekotek.org), a
nonprofit organization in Chicago that aims to make play more accessible for
children with disabilities. Each item is tagged with symbols showing which
abilities the toy may help promote, like social interaction, fine motor skills
or language development.
When Ms. Perry first saw the Toys “R” Us catalog, she felt put off. “It felt
condescending, like we weren’t able to choose the right toys ourselves,” she
said. “Clark’s interests are not that different than other kids.’ ”
But as she examined the catalog more closely and saw the symbols, she realized
that it could be useful. “There are definitely areas Clark needs to work on, and
the symbols show where a toy could help,” she said. “So if it saves me from
spending time and money on the wrong thing, it is valuable.”
Many toys are hard work for children with autism because they stretch the
child’s capabilities in language, social skills or sensory integration. While
these are crucial to the child’s development, Susan Malmquist, director of
educational and clinical services at the autism research center, advises care
providers to balance instructional and therapeutic toys with their favorite
types of toys. If the child loves airplanes, for example, provide them as a
break between more therapeutic activities.
Children with autism usually have a limited set of interest areas, but those
interests are very strong. So whether an interest is in animals, trains or cars,
it is hard to go wrong buying more items in that category.
Many Web sites offer information and products for children with autism and other
special needs. Stars4kidz.com offers toys grouped by development category, like
cause and effect, or sensory play.
Neurodiversity.com, through links to Amazon.com, suggests gifts as well as
children’s books featuring characters with autism, including “Of Mice and
Aliens” by Kathy Hoopmann (Jessica Kingsley Publishers, $12.95) and “Tobin
Learns to Make Friends” by Diane Murrell (Future Horizons, $16.95). The site’s
books for siblings include “All About My Brother” by Sarah Peralta (Autism
Asperger Publishing Company, $16.95).
For parents who want more information about a toy than the mall or the Web might
offer, Discovery Toys (www.discoverytoysinc.com) of Livermore, Calif., will send
a sales representative to make a presentation for a parent’s support group or a
play group. Working with a child development expert, the company has chosen a
variety of toys for children with autism in different skill-building areas. The
Castle Marbleworks Play Tower ($36.99), for example, teaches cause-and-effect
relationships using balls and interlocking plastic ramps.
Ellen Notbohm, author of “Ten Things Every Child With Autism Wishes You Knew”
(Future Horizons, $14.95), advises gift-givers to think beyond the toy store,
especially by emphasizing everyday items that provide sensory experiences. One
year she gave her son with autism a tissue-lined wicker basket containing 10
cans of shaving cream, to be dispensed at will in the bathtub, sink or wading
pool. “He was beyond happy,” she recalled.
Because children with autism often like to play with one type of thing for a
long time, large quantities of typical items can delight them, she said, like a
bucket of flashlights or a treasure chest of costume jewelry collected from
local garage sales.
“Any idea can be terrific, and any idea can be disastrous, depending on the
child,” Ms. Notbohm said. “Parents will appreciate it if you have thought of
three or four suggestions and ask them which might be most appropriate.”
Asking parents in advance is wise because special needs can vary so widely.
Children on the autism spectrum usually have a concrete view of life, so fantasy
characters and toys may not appeal to them. For some, a sense of danger is slow
to develop, so be sure to ask parents about toys with an electrical cord. And
though grandparents may like to shower a child with gifts, one large present may
be better than many small ones. Too many things going on at once, too many
choices, can overwhelm a child with autism, who finds it easier to focus on one
thing at a time. Membership to the local zoo or science museum (where the family
can visit when it is not too noisy or crowded) is another possible gift.
Most buying guidelines for children with autism are the same as those for any
other children, according to Dr. Malmquist at the autism research center. Find
toys based on their interests, toys that will help them develop and toys that
are safe and matched to their intellectual capabilities. It may just take a
little more research.
For
the Autistic, a Gift of Common Ground, NYT, 26.11.2006,
http://www.nytimes.com/2006/11/26/business/yourmoney/26disable.html
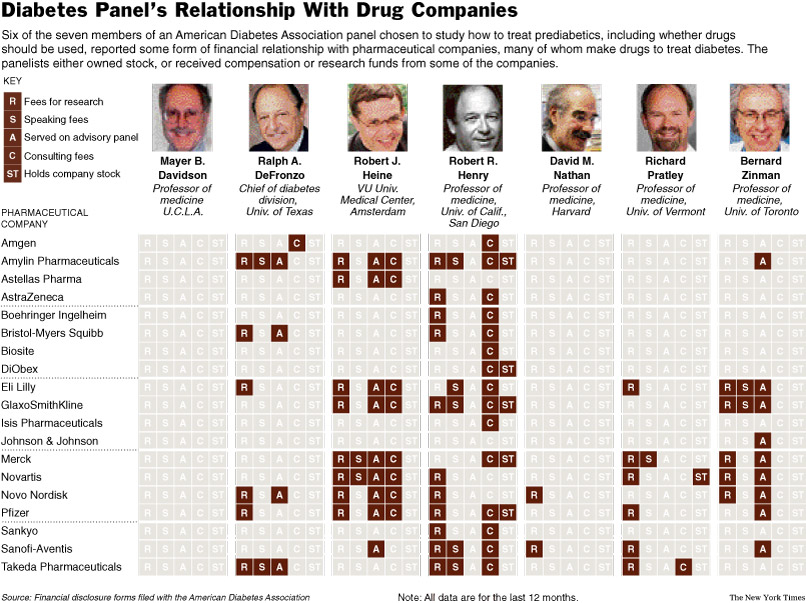
In Diabetes Fight, Raising Cash and
Keeping Trust NYT
25.11.2006
http://www.nytimes.com/2006/11/25/health/25ada.html?hp&ex=
1164517200&en=11f7cde2ce1b51ee&ei=5094&partner=homepage
In Diabetes Fight,
Raising Cash and Keeping Trust
November 25, 2006
The New York Times
By MARC SANTORA
SnackWell’s Sugar-Free Lemon Creme cookies
have nearly as many calories as some sugar-rich cookies. Yet until recently the
box featured an American Diabetes Association logo, advertising the cookie as a
“proud sponsor” of the charity’s efforts on behalf of the nation’s 21 million
diabetics.
Foods like the Sugar-Freedom Eskimo Pie and Frosted Shredded Wheat have also
sported the American Diabetes Association logo over the years. The companies
paid the A.D.A. to be associated with a respected voice for healthful eating.
The association wanted the money to finance its uphill battle against a widening
epidemic of Type 2 diabetes, which is associated with obesity.
But in the last year the A.D.A. began rethinking how it raises money from
companies, especially from those whose primary business is selling foods and
beverages that are high in calories, even if they have created some sugar-free
items.
The group has allowed some food company deals to expire and has turned down
millions of dollars in new sponsorships.
Many public health charities, from the American Heart Association to the Lupus
Foundation of America, raise money from businesses. But for the A.D.A., and some
other charities, the effort has increasingly become an exercise in balancing the
need to raise money with core matters of conscience.
“We tightened things up,” Dr. Richard Kahn, a top A.D.A. executive, said of the
association’s new guidelines for corporate fund-raising, “because we were
beginning to be bombarded by all kinds of food companies selling all kinds of
products with requests to be a ‘proud sponsor’ or to advertise.”
Some consumer and food activists say the guidelines, while good, do not go far
enough.
They say the A.D.A. remains too wedded to benefactors in the food and
pharmaceutical industries, who provided more than $23 million last year.
Of particular concern: a three-year, $1.5 million sponsorship deal with
Cadbury-Schweppes, the world’s largest confectioner. Under the deal, which meets
the new guidelines, Cadbury is promoted as an A.D.A. sponsor in several
settings, and has permission to use the A.D.A. logo on its Diet-Rite sodas,
Snapple unsweetened tea and Mott’s Apple Sauce, among other products.
Critics say the A.D.A. affiliation has helped Cadbury pose as a concerned
corporate citizen, even as it supplies grocery stores with sugary and fattening
foods like Dr Pepper and the Cadbury Creme Egg.
“Maybe the American Diabetes Association should rename itself the American Junk
Food Association,” said Gary Ruskin, director of Commercial Alert, a consumer
advocacy group.
Others remain concerned about the A.D.A.’s relationships with pharmaceutical
companies. Their presence is evident throughout the charity, from its annual
convention, which is largely underwritten by drug makers, to its board meetings,
where pharmaceutical executives have served on the volunteer committees that set
policy.
The A.D.A. says its independence is evident because it has often acted against
the interests of the pharmaceutical industry. Last month, for example, a panel
it appointed to study how to treat people at heightened risk of developing
diabetes decided against recommending the use of higher-priced brand-name drugs.
But critics say the drug industry’s influence can be seen in the A.D.A.’s
emphasis on the treatment of diabetics, which often involves drug therapy, over
efforts to persuade people to change the way they live so that the disease can
be prevented in the first place.
“I’m glad the A.D.A. finally took its logos off of sugary cereals,” said Marion
Nestle, a professor of nutrition and food studies at New York University.
“Nevertheless, the A.D.A. still needs to pay more attention to prevention. Right
now, it puts way more resources into treatment.”
Companies say they give to the A.D.A. because they share the goal of helping
people lead healthier lives. Drug makers say their products work hand in hand
with A.D.A. efforts to better treat diabetics. Food company executives say they
hope to give consumers greater choice by drawing attention to new, healthier
products, which are increasingly popular.
The A.D.A., more than many other charities, already has a tough time raising
money. Type 2 diabetics, frequently old and overweight and judged by some as
partly responsible for their own plight, do not generate as much sympathy, or
contributions, as many other sick people.
Faced with financial pressures, A.D.A. officials say they try to focus on
practical solutions, like treatment options, more than lifestyle modifications.
It is not favoritism toward pharmaceutical companies, as some suggest, Dr. Kahn
said. It is skepticism toward the notion that many Type 2 diabetics have the
discipline to eat less and exercise more.
“Ninety percent of the people out there still can’t lose 10 percent of their
body weight and keep it off for four years,” he said. Nonetheless, he said, the
A.D.A. will continue education efforts to reverse the tide of obesity.
With the emergence of Type 2 diabetes as the nation’s fastest growing health
problem, increasing by 80 percent in the last decade, this is an important time
for the A.D.A. The 66-year-old charity, based in Alexandria, Va., is the primary
coordinator for the national response to the disease and directs much of the
lobbying, treatment guidelines and education efforts that address the major
forms: Type 1, a genetic disorder that usually surfaces in children, and Type 2,
a far more prevalent illness in which genetics plays a role, but in which
obesity and inactivity are the key risk factors.
Each year, diabetes causes hundreds of thousands of Americans to suffer heart
failure, lose limbs, go blind, or die. Yet even with corporate support and
substantial grass-roots fund-raising, the A.D.A.’s $210 million budget is
stretched by its many missions: educating legislators, publishing medical
journals, running camps for diabetics across the country.
From the A.D.A.’s perspective, no charity is more rigorous about ethical
standards or more transparent about where its executives earn and invest their
money. Even unpaid volunteers must detail their income and financial holdings.
“I want to be able to sleep at night,” said Vaneeda Bennett, the A.D.A.’s chief
fund-raiser, “knowing that we are doing the right thing for people with
diabetes, not doing anything that could, in any way, jeopardize that trust.”
Dr. Peter Lurie, deputy director of the Health Research Group of Public Citizen,
the government watchdog group, said the influence of pharmaceutical money can be
very subtle.
“The question is what happens in the close calls,” he said. “If you are at more
cocktail parties, if you have more mugs from the company in your kitchen, you
are just going to be more receptive.”
A.D.A. officials cited an event from several years ago to illustrate their
resistance to such influence. An A.D.A. panel found that antipsychotic drugs
could help fuel diabetes. The announcement angered a drug manufacturer, Eli
Lilly and Company, a longstanding A.D.A. benefactor that stood to lose hundreds
of millions of dollars in lawsuits.
Some A.D.A. officials said they believed the company became so angry it sought
to have Dr. Kahn fired, a charge the company denies.
Either way, nothing happened. Dr. Kahn, the association’s chief scientific
officer, kept his job.
“Show me one instance where money has caused us to do something that is wrong,”
Dr. Kahn said in an interview. “You can’t.”
Corporate Friends
When the A.D.A.’s executive committee met at the W Hotel in Seattle in 2003, a
special handout circulated around the room.
It outlined a business deal so sensitive it had not been listed on the official
agenda and the handouts were collected at the meeting’s end.
Two words on the handout stood out.
Burger King.
Even within an organization that had made deals with food companies, the
fast-food business was different.
Like McDonald’s, Burger King had become a lightning rod for criticism of junk
food and its impact on childhood obesity.
But Burger King told the A.D.A. that it was coming out with more healthful
products, like a grilled sandwich and a side of vegetables, and hoped the
charity would consider a partnership.
“They assured us that this was not just something they were doing to respond to
the criticism over their role in the obesity epidemic,” said Nancy
Stinson-Harris, the A.D.A.’s managing director for corporate alliance and
cause-related marketing.
To review the A.D.A.’s dealings with Burger King is to understand its struggle
to raise money and preach health without crossing lines or demonizing foods.
“There are some within A.D.A. who say no fast food whatsoever and there are some
that say that eating healthy is about choice and people need to make good
choices,” said Ms. Bennett. “We want to embrace the healthy choices and work
from within the tent.”
For the A.D.A., working within the tent has meant choosing persuasion over
protest. Critics say the organization is too silent on vanguard issues like
special taxes on foods like soda. But the A.D.A. says it will take more than
shouting to change America’s eating habits. It will take focused efforts to prod
mainstream manufacturers toward offering healthier products.
To that end, the association signed deals with more than a dozen food companies
over the years, including Bird’s Eye, Campbell’s, General Mills and Coca-Cola.
Each deal was distinct, but generally the companies financed an A.D.A. health
campaign and were recognized as sponsors. Some received permission to use the
A.D.A. logo on select products, generally sugar-free and diet brands.
Kraft, for example, as part of a four-year, $1 million deal that expired this
year, was recognized as an A.D.A. sponsor and could post the A.D.A. logo on
products like SnackWell’s cookies, Post Raisin Bran, Cream of Wheat cereal and
Sugar-Free Jell-O.
In some cases, the products that had permission to use the logo were only
slightly healthier than those that did not.
Kraft, for example, never used the logo on Cool Whip Lite, but it had
permission, even though the topping had just five fewer calories per serving
than regular Cool Whip.
In Burger King’s case, negotiations toward a deal stretched on for nearly a
year. Then the A.D.A. learned that the company was coming out with another new
product. “It was a burger that would have the most fat that has ever been on the
market,” Ms. Stinson-Harris recalled. Burger King did not respond to several
requests for comment.
The A.D.A. dropped negotiations at that point. But officials of the charity
seized the opportunity to create a more formal set of policies about corporate
fund-raising. The guidelines installed this year are 54 pages long and cover the
gamut of corporations, from food manufacturers to drug makers.
“The association,” they say, “should refrain from associating with companies
that have the potential to damage A.D.A.’s image because of the nature of the
companies’ products, services or reputation.”
Because of the guidelines, several deals with food companies that expired could
not be renewed, officials said. Hershey and the A.D.A., for example, could not
come to terms on renewing an agreement, worth more than $200,000 a year. A.D.A.
officials said they would not agree to the use of their logo on a sugar-free
chocolate candy because of its fat content.
“Now the first thing we do is look at the content, the label,” Ms. Bennett said.
“In the past we didn’t. The fat was not a consideration.”
The A.D.A., which took in more than $3 million from food companies last year,
has not signed a new agreement with a national food company since the guidelines
took effect. One obstacle is that the guidelines set a minimum contribution of
$500,000 for any company that would seek permission to use the A.D.A. logo, a
new provision borne of the organization’s belief that an association with its
name has undeniably high value.
“There are still some people who are pained by the fact that we cannot do a deal
on this or that because of the guidelines,” said Dr. John Buse, the incoming
A.D.A. president.
Though they often present the most difficult choices, food companies represent a
small segment of the A.D.A.’s corporate support. Pharmaceutical companies remain
the largest corporate contributors, but the guidelines have not affected them as
much because the A.D.A. has never allowed its logo to be put on specific
medicines.
And for all the scrutiny the new guidelines are said to have brought, critics
are quick to note that they did nothing to block the A.D.A. from doing business
with Cadbury, a $12 billion-a-year empire built on the consumption of sweets.
“This isn’t really rocket science,” said Mr. Ruskin, the consumer activist.
“This is damage to their integrity. And this is a serious matter.”
Cadbury officials say their association with the A.D.A. is a good-faith effort
to promote health. At one point last year, Dr. Kahn also tried to defend the
Cadbury deal by telling an online publication: “There is not a shred of evidence
that sugar, per se, has anything to do with diabetes.” To Dr. Kahn it was a
matter of basic science. Though diabetics should watch their sugar intake, many
foods, not just sugar, he said, carry the kinds of calories that can lead to
obesity and ultimately diabetes.
But the remark struck some as careless, given concerns about excess sugar in
many diets.
Ms. Bennett acknowledged that negotiating deals like the Cadbury agreement are
difficult “when a good company offers several ‘good for you’ as well as ‘bad for
you’ products.” In the end, she said, Cadbury improved the deal by agreeing to
use its own advertising budget to promote the A.D.A.’s new Weight Loss Matters
program.
More than a year into the arrangement, the A.D.A. says it is happy with how it
turned out. At a meeting this fall, it honored Cadbury with a corporate
recognition award.
Valuable Advice
At a Chicago hotel last month, the A.D.A. brought together seven medical
experts, widely viewed as stars in their field, to consider an issue that could
radically alter the way people at heightened risk of developing diabetes are
treated. It also had bearing on the fortunes of at least one A.D.A. benefactor.
For years, doctors have debated whether to use drugs to treat people who are
prediabetic, meaning their blood sugar is elevated, but not to the level
considered diabetic.
An estimated 41 million people in America are viewed as prediabetic, in danger
of developing the disease. One drug manufacturer, GlaxoSmithKline, recently
completed trials of a new drug to treat this potentially huge market and, in
several months, the A.D.A. panel will publish its recommendation on whether drug
treatment for prediabetes is warranted.
A.D.A. rules prohibit such panels from reviewing the efficacy of an individual
brand. But the A.D.A.’s perspective on whether a class of drugs should be used
in treatment often carries great weight with the Food and Drug Administration,
which must approve the Glaxo drug for use, and with doctors who write
prescriptions.
So the appointed panel’s perspective is likely to be closely watched by Glaxo,
which donated more than $1 million to the A.D.A. last year.
All but one member of the panel reported receiving fees or research funding from
pharmaceutical companies on their A.D.A. financial disclosure forms. Three
reported direct compensation from Glaxo, though no amounts were listed.
Few in medical care believe such payments disqualify experts from serving on
panels. Many research scientists receive such funding.
They are, nonetheless, representative of the sort of complications that arise
when the work of charities and corporations become significantly intertwined.
The A.D.A.’s relationship with drug companies dates to 1940, when 26 doctors met
at Schrafft’s Restaurant in Manhattan to create the association, using a $1,000
gift from the drug maker Eli Lilly. Twice in the past decade, the A.D.A.’s
12-member Executive Committee has been led by the former top executives of drug
or medical equipment companies. Today the A.D.A.’s treasurer is the director of
investor relations for Johnson & Johnson.
Pharmaceutical companies sell $15 billion worth of diabetes drugs in the United
States each year and the A.D.A. is now a fixture in their marketing strategy.
The companies advertise in A.D.A. journals and announce new medicines at A.D.A.
conventions, where a coming-out party for a touted new drug can drive a stock
higher.
The convention, known as the Annual Scientific Sessions, routinely draws some
15,000 doctors, health care workers and experts to a conference center where
everything from the water bottles to the chartered buses are plastered with ads
from drug companies.
“It’s not quite brainwashing, but they have a way of influencing your thinking,”
Dr. Buse said of the companies. He said some companies sent hundreds of
employees to the convention, nearly all of them there to promote new medicines.
Dr. Buse said he did not believe that the money, or the manpower, ultimately
influences A.D.A. policy. But he said the level of fraternization made him
uncomfortable, and might hurt the A.D.A.’s image. He said it was one of the more
pressing issues facing public health charities, who have come to rely on
pharmaceutical money for a large portion of their budget.
“It is a real problem,” he said.
One of its members of the A.D.A. panel studying prediabetes, Dr. Ralph A.
DeFronzo, chief of the diabetes division at the University of Texas Health
Science Center, said the panel had decided to recommend lifestyle interventions
and some use of a generic drug for prediabetics, but had stopped short of
advocating the use of newer, brand-name drugs, such as the one being developed
by Glaxo.
The decision can potentially affect billions of dollars in sales because the
prediabetic market is so large and the difference in price so great between
generic and “branded” drugs. The generic drug the panel recommended, for
example, metformin, costs about $240 a year, just a fifth to a tenth of what a
branded drug prescribed in the same setting might cost, experts said.
“I think the panel’s recommendation was really quite conservative,” Dr. DeFronzo
said.
In the past year, Dr. DeFronzo has received compensation from seven drug
companies, though not Glaxo. The companies either financed his research or paid
him for work as a speaker or as a consultant, according to his disclosure form.
He said he was confident that such support never influenced his judgment.
“To be honest,” he said, “if you are the best person in the world, why wouldn’t
a company, why wouldn’t the A.D.A., want you on a panel?”
Dr. Kahn said that, even with extensive safeguards, he and his staff ultimately
must rely on the integrity of the people they appoint. It becomes an act of
faith, and like all acts of faith, he said, there may well be nonbelievers when
the panel’s report comes out.
“We have no choice,” he said. “There will always be a few people who think that
we are biased.”
In
Diabetes Fight, Raising Cash and Keeping Trust, NYT, 25.11.2006,
http://www.nytimes.com/2006/11/25/health/25ada.html
NYT
November 22, 2006
Proof Is Scant on Psychiatric Drug Mix for
Young NYT
23.11.2006
http://www.nytimes.com/2006/11/23/health/23kids.html
Proof Is Scant
on
Psychiatric Drug Mix for Young
November 23, 2006
The New York Times
By GARDINER HARRIS
Their rooms are a mess, their trophies line the walls, and
both have profiles on MySpace.com. Stephen and Jacob Meszaros seem like typical
teenagers until their mother offers a glimpse into the family’s medicine
cabinet.
Bottles of psychiatric medications fill the shelves. Stephen, 15, takes the
antidepressants Zoloft and Desyrel for depression, the anticonvulsant Lamictal
to moderate his moods and the stimulant Focalin XR to improve concentration.
Jacob, 14, takes Focalin XR for concentration, the anticonvulsant Depakote to
moderate his moods, the antipsychotic Risperdal to reduce anger and the
antihypertensive Catapres to induce sleep.
Over the last three years, each boy has been prescribed 28 different psychiatric
drugs.
“Sometimes, when you look at all the drugs they’ve taken, you wonder, ‘Wow, did
I really do this to my kids?’ ” said their mother, Tricia Kehoe of Sharpsville,
Pa. “But I’ve seen them without the meds, and there’s a major difference.”
There is little doubt that some psychiatric medicines, taken by themselves, work
well in children. For example, dozens of studies have shown that stimulants
improve attentiveness. A handful of other psychiatric drugs have proven
effective against childhood obsessive compulsive disorder, among other problems.
But a growing number of children and teenagers in the United States are taking
not just a single drug for discrete psychiatric difficulties but combinations of
powerful and even life-threatening medications to treat a dizzying array of
problems.
Last year in the United States, about 1.6 million children and teenagers —
280,000 of them under age 10 — were given at least two psychiatric drugs in
combination, according to an analysis performed by Medco Health Solutions at the
request of The New York Times. More than 500,000 were prescribed at least three
psychiatric drugs. More than 160,000 got at least four medications together, the
analysis found.
Many psychiatrists and parents believe that such drug combinations, often
referred to as drug cocktails, help. But there is virtually no scientific
evidence to justify this multiplication of pills, researchers say. A few studies
have shown that a combination of two drugs can be helpful in adult patients, but
the evidence in children is scant. And there is no evidence at all — “zero,”
“zip,” “nil,” experts said — that combining three or more drugs is appropriate
or even effective in children or adults.
“There are not any good scientific data to support the widespread use of these
medicines in children, particularly in young children where the scientific data
are even more scarce,” said Dr. Thomas R. Insel, director of the National
Institute of Mental Health.
Psychiatrists who prescribe drug combinations say that the ability to mix and
match medications improves their chances of being able to help children who are
seriously, even desperately, ill.
Dr. Joseph Biederman, a professor of psychiatry at Harvard, said that doctors
commonly used multiple medicines to treat heart disease, diabetes, cancer and
AIDS. “Child psychiatry is not any different,” Dr. Biederman said. “These drugs
have revolutionized how we treat severe psychopathology in children.”
The controversy leaves parents in a terrible bind. Desperate to help, many
agonize over whether to medicate their children.
Mothers and fathers sometimes disagree, with the dispute straining or even
ending marriages. Since some psychiatric drugs can cause worrisome physical
effects, parents say that they must on occasion make a terrifying choice between
their child’s physical health and his mental health.
The parents interviewed for this article told their stories, they said, in hopes
of gaining greater acceptance for their children and themselves. Nearly all
recalled being in a store when their child threw a tantrum and feeling that
onlookers branded them as bad parents. They also said they hoped to help others
negotiate what many said were unequal and often fraught relationships with
psychiatrists.
“We struggled so much, made so many mistakes and felt so stigmatized, I hope our
story can make it easier for others,” said Jacquie Erickson of Anchorage. Her
daughter, Kaitlyn Johnston, 10, has taken psychiatric drugs since she turned 5
for diagnoses that include bipolar disorder.
On Shaky Ground
Stimulants like Ritalin are by far the most commonly prescribed psychiatric
medicines in children. But doctors routinely pair stimulants with
antidepressants, antipsychotics and anticonvulsants, even though some of these
medications can cause serious side effects, have few proven pediatric
psychiatric benefits and lack clear evidence about how they interact or
influence mental and physical development.
Last year, the Food and Drug Administration required drug makers to warn on
their labels that antidepressants can cause suicidal thoughts and behavior in
some children. Anticonvulsant drugs carry warnings about liver and pancreas
damage and fatal skin rashes. The side effects of antipsychotic medicines can
include rapid weight gain, diabetes, irreversible tics and, in elderly patients
with dementia, sudden death. When drugs are combined, these risks compound.
Ms. Kehoe, who receives government financial and child-care assistance because
her children are considered mentally ill, said she knew that there were risks to
the drug cocktails. Both her sons are short and underweight for their age — a
common side effect of stimulants — and she fears that the drugs have affected
their health and behavior in other ways.
“But I don’t think the insurance would pay for it if the F.D.A. didn’t decide
that children should use it,” said Ms. Kehoe, who herself takes psychiatric
medication.
In fact, the drug agency has specifically warned against the use of Lamictal,
one of the drugs Stephen takes, in children who, like him, do not suffer from
seizures because in 8 out of 1,000 children the drug causes life-threatening
rashes.
Stephen and Jacob’s psychiatrist did not reply to telephone messages left with
an office secretary on three different days. Ms. Kehoe said that she asked him
to speak to this reporter but that he refused. The boys have had 11
psychiatrists over the last three years, according to prescription records, and
many more before that, Ms. Kehoe said.
In interviews, Stephen and Jacob said they hated taking their drug cocktails.
“Everybody hates meds,” Jacob said.
Ms. Kehoe said her youngest son, Lucas Keck, was showing signs of attention
deficit disorder and might soon need to start medication.
“I see the hyperness in him,” she said. “My pediatrician has said that he would
venture to say that Lucas will be A.D.H.D.”
Stephen and Jacob were Lucas’s age — 6 — when they were given their first
prescriptions.
The F.D.A. requires drug makers to prove that their drugs work safely before the
agency will approve them for sale in the United States. But doctors can
prescribe and combine approved medicines as they see fit. Such mixing is common
in medicine but rarely studied by drug makers.
Psychiatrists started mixing psychiatric medications because the drugs were only
moderately effective and often caused terrible side effects, said Dr. Steven E.
Hyman, the provost of Harvard University and former director of the National
Institute of Mental Health. “None of these drugs by themselves do an adequate
job of controlling symptoms,” Dr. Hyman said.
If one drug failed, many psychiatrists assumed that two or more drugs used
together might succeed. For decades, no one studied whether this was accurate.
But in recent years, a trickle of studies have examined the question, with mixed
results.
In studies in adults, some combinations of two drugs have been shown to work
better than single medications to improve the symptoms of depression,
obsessive-compulsive disorder and the mania associated with bipolar disorder.
For example, a recent large government-financed study in adults, published in
The New England Journal of Medicine, found that two antidepressants worked a bit
better than one for adults who suffered from chronic, severe depression. But
other studies have found no benefit from commonly prescribed drug combinations.
The use of two-medicine combinations in children is on much shakier ground. Even
for single drugs, the effectiveness of some psychiatric medications in younger
patients is questionable: most trials of antidepressants in depressed children,
for instance, fail to show any beneficial effect. But hardly any studies have
examined the safety or the effectiveness of medicine combinations in children. A
2003 review in The American Journal of Psychiatry found only six controlled
trials of two-drug combinations. Four of the six failed to show any benefit; in
a fifth, the improvement was offset by greater side effects.
“No one has been able to show that the benefits of these combinations outweigh
the risks in children,” said Dr. Daniel J. Safer, an associate professor of
psychiatry at Johns Hopkins University and an author of the 2003 review.
If the evidence for two-drug combinations is minimal, for three-drug
combinations it is nonexistent, several top experts said.
“The data is zip,” Dr. Hyman said.
Many psychiatrists said that they turned to drug cocktails only in desperate
circumstances. “If you’ve got a 15-year-old who is cutting up her arms, you’ve
got a barn on fire and what are you supposed to do?” asked Dr. Alexander Lerman,
a child and adolescent psychiatrist in New York, who said he rarely prescribed
combinations.
Billy and Jackie Igafo-Te’o of Jackson, Mich., are among the desperate. In the
last seven years, their 12-year-old son, Michael, “has been on just about
everything you can put a child on,” Mrs. Igafo-Te’o said. He is now taking four
medications: an antipsychotic, an anticonvulsant, an antidepressant and a sleep
medicine.
Despite the medications, Michael’s behavior has grown increasingly disruptive.
He has kicked and punched holes in almost every wall of the Igafo-Te’o home. He
wrenched the sink off the wall in the upstairs bathroom and pulled two bedroom
doors off their hinges, damaging the frames. The family no longer fixes the
damage.
During a recent visit, Michael and Mr. Igafo-Te’o were sitting on the
living-room floor. Michael wanted the phone. His father held it out of reach to
prevent Michael from playing with it. Michael became increasingly desperate. He
cried. He cursed.
“That’s it, you have a timeout,” Mr. Igafo-Te’o said.
“No, no, no,” Michael answered. “You pimp!”
He slapped his father in the face, hard. Mr. Igafo-Te’o hustled Michael into the
kitchen and forced him to sit for 20 minutes.
“What’s the purpose of all this medication if I still have to do that?” Mr.
Igafo-Te’o asked.
He said he wanted to end Michael’s drug therapy. Among other side effects, the
drugs have made Michael obese, which has led to asthma.
Mrs. Igafo-Te’o quietly disagreed. “I’m afraid he wouldn’t be able to focus,”
she said. “I’m afraid he would regress socially.”
“Regress socially? Look at him!” her husband responded, motioning to their son,
crying uncontrollably on the kitchen floor.
“I have to believe in something,” his wife mumbled and walked out of the room.
Mr. Igafo-Te’o watched her go and then smiled apologetically.
“We always debate meds,” he said.
Divergent Views
Most experts agree that some children are so violent or suicidal that a
combination of psychiatric drugs is worth trying. But recently, more
psychiatrists have been asking whether in some cases drugs are being prescribed
for children who do not need them, or for problems that fall within the spectrum
of normal behavior. The doubters are especially concerned with the growing use
of drug combinations for preschoolers.
Fate Riske, 3, of Fond du Lac, Wis., takes two antipsychotics and a sleeping
medicine to control what her mother, Elizabeth Klein-Riske, said were hours-long
tantrums, a desire to watch the same movies repeatedly and an insistence on
eating the meat, cheese and bread in her sandwiches separately.
On a recent visit, Fate played sweetly for four hours as her parents, who both
have trouble walking, sat in front of a television. Sucking on a pacifier, Fate
showed off her pink dress and matching shoes.
Mrs. Klein-Riske credited the drugs for Fate’s cherubic behavior during the
visit. But a few weeks on a different antipsychotic led Fate to become
aggressive, talk rapidly and “run around wild, totally out of control,” said
Mrs. Klein-Riske, who receives government financial and child-care assistance
because her daughter is considered mentally ill.
Fate’s weight ballooned in five months to 48 pounds from 30.
Dr. Gary Sachs, director of the Bipolar Clinic and Research Program at
Massachusetts General Hospital in Boston, estimated that half the children
referred to his clinic for research in recent years — including many who took
drug combinations — had the wrong diagnosis and often did well on fewer drugs.
“Even among properly diagnosed bipolar patients, many come to our program
already taking medicines that interfered with each other,” Dr. Sachs said.
But Dr. Judith Rapoport, a senior investigator in child psychiatry at the
National Institute of Mental Health, said that in her experience, few children
were overmedicated. Dr. Rapoport studies children with schizophrenia. Before
entering her study, children must be drug-free for three weeks.
“We’ve had a handful of cases who are completely normal when they get off
drugs,” Dr. Rapoport said. “But most of these kids become very, very sick and
unmanageable without drugs.”
The first psychiatric problem diagnosed in most children is attention deficit
disorder, treated with stimulants — drugs that improve attentiveness. But when
children’s problems persist, parents’ relatively good experience with stimulants
often convinces them to agree to try other medicines — in some cases drugs like
the antipsychotic Risperdal or the anticonvulsant Depakote that have few proven
benefits in children and greater dangers, said Dr. Ranga Krishnan, chairman of
the department of psychiatry and behavioral science at Duke University.
“After you get them on one drug, parents don’t seem to mind the second,” said
Dr. Krishnan, who said that he had grave doubts about the growing use of
psychiatric drug cocktails in children.
Antidepressants are commonly paired with stimulants, but antidepressant use has
declined over the last year after the F.D.A. warning about suicide risk. In
their place, physicians are prescribing combinations that include antipsychotic
and anticonvulsant drugs, according to Medco. From 2001 to 2005, the use of
antipsychotic drugs in children and teenagers grew 73 percent, Medco found.
Among girls, antipsychotic use more than doubled.
On Again, Off Again
Andrew Darr of Caldwell, Idaho, whose sons took medications, said that he was
opposed to it from the start. “When you come home from work and instead of
getting them clawing at your feet and yelling, ‘Daddy, Daddy,’ you get a
lethargic grunt, it just kills you,” Mr. Darr said.
His wife, Leslie Darr, eventually agreed to stop the medicines, but only after a
family tragedy.
The Darrs have four children, Nicholas, 16, Nathan, 15, Becky, 12, and Benjamin,
9. At 3, Nicholas suffered a mild brain injury when undiagnosed appendicitis led
him to suffer weeks of high fever, Mrs. Darr said.
Mrs. Darr said that she was pressured by school officials to give Nicholas a
stimulant at age 6. Nathan soon followed.
Three years later, the boys had a traumatic weekend away with relatives. A month
after that, Mrs. Darr said, both were hospitalized for a week and given a
diagnosis of bipolar disorder and prescriptions for antipsychotic,
antidepressant and sleeping medicines.
Over the next three years, Nicholas’s weight ballooned to 140 pounds from 52.
Nathan went to 115 pounds from 48. Neither boy got much taller, Mrs. Darr said.
They did poorly in school.
Then Becky developed a brain tumor. A nurse practitioner gave Mrs. Darr free
samples of an antipsychotic drug to help her cope. After starting it, she said,
she could not sleep or think straight. She realized that she had been giving
similar medicines to her sons for years and she decided to wean the boys off the
pills.
Their behavior immediately worsened. At one point, Nicholas left the house
during a blizzard wearing only boxer shorts, Mrs. Darr said. They found him in a
tire swing saying, “Baaa.”
“There were several times that we almost gave up,” Mr. Darr said.
But after four months off medication, the boys’ behavior normalized, the Darrs
said, and they were transferred out of special education and into regular
classes. The Darrs recently allowed the boys to spend their first evening at a
mall without supervision, and in July they gave both boys their first bicycles.
“They’ve come a long way,” Mrs. Darr said.
In an interview, Nicholas said the drugs “were not cool.”
“You go to school and everybody thinks, ‘Look at that retard,’ ” he said.
Still, most of the parents interviewed for this article said their children’s
behavior deteriorated rapidly without medication.
Joanne Johnson of Hillsborough, N.J., described a psychiatrist’s effort to wean
her 17-year-old son, Brad, off of all five of his psychiatric medicines as “the
biggest mistake of our lives.”
Brad, then 13, became suicidal and was hospitalized for weeks, Ms. Johnson said.
“He went into the hospital on five drugs and came out on five different ones,
but he was unstable,” she said. “It took a little over two years to find the
right match again.”
Brad is now taking lithium, an antipsychotic, an anticonvulsant, an
antidepressant, a stimulant and a sleeping pill.
“He’ll probably be on these for the rest of his life,” Ms. Johnson said.
Proof Is Scant on
Psychiatric Drug Mix for Young, NYT, 23.11.2006,
http://www.nytimes.com/2006/11/23/health/23kids.html?hp&ex=1164344400&en=266fbd110aa0d9cf&ei=5094&partner=homepage
Journal Clarifies Report on a Stem Cell Finding
November 23, 2006
The New York Times
By NICHOLAS WADE
The scientific journal Nature has issued a clarification of
a recent report that human embryonic stem cells can be derived without harm to
the embryo, but has affirmed the report’s validity.
The finding, by Dr. Robert Lanza and colleagues at Advanced Cell Technology’s
laboratory in Worcester, Mass., caused a stir when Nature published it online in
August, because under current methods, the generating of such cells entails
destroying the embryo. The paper would seem, then, to undercut the argument of
opponents of embryonic stem cell research that the work requires the loss of a
potential human life.
Dr. Lanza’s approach is made possible by preimplantation genetic diagnosis, a
test sometimes used in fertility clinics whereby one cell is removed to test for
abnormalities when the embryo has reached the eight-cell stage. This does no
apparent harm to the embryo, which, if the testing finds it normal, is then
implanted with its seven remaining cells. The process has resulted in the birth
of apparently healthy children.
Dr. Lanza found that one such cell, or blastomere, could generate embryonic stem
cells. His article is being published again by Nature — online yesterday and in
the printed journal today — along with itemized changes in the text and an
addendum by him and his colleagues.
The changes address issues that were misunderstood or misstated earlier, as in a
press release issued by Nature. These included the fact that although Dr. Lanza
said others could use his method to avoid destroying embryos, he himself had
destroyed those used in his experiments by using many cells from each. Had he
taken just one cell from each of a number of eight-cell embryos, many more
embryos would have been needed.
Ritu Dhand, Nature’s chief biology editor, said the sole purpose of the
clarification was to make the article easier to understand. “There was nothing
either scientifically or technically wrong with the paper,” Dr. Dhand said. The
journal has had the article reviewed by experts a second time, she said, “just
to be sure that everything was as it should be.”
It is too soon for other laboratories to have repeated Dr. Lanza’s work, the
acid test of scientific findings.
Richard M. Doerflinger, a spokesman for the United States Conference of Catholic
Bishops, said the ability to derive stem cells from a single blastomere was
still unproved.
Journal Clarifies
Report on a Stem Cell Finding, NYT, 23.11.2006,
http://www.nytimes.com/2006/11/23/science/23stem.html
F.D.A. Will Allow Breast Implants Made of Silicone
November 18, 2006
The New York Times
By STEPHANIE SAUL
The Food and Drug Administration yesterday lifted a 14-year
ban on the use of silicone gel breast implants in the United States after
decades of contentious debate and litigation over their safety.
The federal agency approved implants manufactured by two California companies,
Mentor and Allergan, for breast reconstruction and cosmetic breast augmentation,
but limited cosmetic use of the implants to women ages 22 and older.
The decision appeared to end a controversy over the safety of silicone implants
that lasted more than two decades and resulted in thousands of lawsuits by women
who claimed the implants leaked and caused a number of diseases, including
cancer and rheumatoid arthritis. The dispute led to the bankruptcy of the
manufacturer Dow Corning, a federal moratorium on the use of the implants, and,
finally, findings by both the Institute of Medicine and the Food and Drug
Administration that the devices do not cause major illnesses.
Because the implants made of silicone gel are softer than the saline implants
currently available, plastic surgeons said they would quickly become preferred
among the more than 300,000 women in this country who have breast implants each
year.
Critics of the decision lambasted it and said that longstanding safety concerns
had not been resolved. But supporters of the implants, including leading
surgeons, applauded it.
“For us, it’s a triumph of science,” said Dr. Richard A. D’Amico of Engelwood,
N.J., president-elect of the American Society of Plastic Surgeons. “We’ve always
felt that the science would bear out the use of the implants.”
Dr. Daniel G. Schultz, director of the F.D.A.’s Center for Devices and
Radiological Health, said that the agency’s review, based on company-sponsored
studies as well as long-term use of the implants abroad, had determined that
their sale is in the best interest of women.
But Dr. Schultz warned that no device is foolproof and that there was a
possibility that women would have to have the implants replaced at some point,
sometimes because they rupture. Studies have found that the majority of women
with silicone implants would have a rupture at some point. According to the
federal agency, one study found that 69 percent of women had a rupture.
“Women should know that breast implants are not lifetime devices,” he said in a
telephone briefing for reporters last night.
“Women having these procedures done need to be prepared for the fact that there
is a likelihood they will require additional surgery,” he said.
He also recommended regular M.R.I.’s to monitor the devices for “silent
rupture,” which can occur without a woman’s knowledge. He said the first M.R.I.
should be performed when the implants are 3 years old. Because many of the
procedures are cosmetic, it was not clear whether those M.R.I.’s would be
covered by insurance.
Critics of the agency said yesterday that the devices should not have been
approved, and cited the same safety concerns that have dogged the devices for
years.
Dr. Sidney Wolfe, chief of Public Citizen’s Health Research Group, which claimed
in the 1980s that breast implants cause cancer, called the implants “the most
defective medical device ever approved by the F.D.A. The approval makes a
mockery of the legal standard that requires ‘reasonable assurance of safety.’ ”
Amy Allina, program director at the National Women’s Health Network, also
sharply criticized the decision, saying that the federal agency had approved the
implants even though the manufacturers had failed to answer basic safety
questions, such as exactly how long implants would last without rupturing, and
whether there would be health effects if the silicone leaked out and traveled to
other parts of the body.
She predicted that the companies would begin a “massive, massive marketing
campaign,” and that women might be taken in by it and assume that the F.D.A.’s
stamp of approval means implants are safe.
Mentor Corporation of Santa Barbara, Calif., which calls its product Memory Gel,
had already initiated one Web site last night to advertise the product:
Mentor4Me.com. The site has a function allowing patients to find a plastic
surgeon.
Defending the decision to lift the ban, Dr. Schultz said, “We have been looking
at this data continuously for the last 10 years. We have been watching as data
had been collected, we have been watching as data has accumulated. We believe
that from a scientific standpoint, the decision that we’re making tonight is, in
fact, in the best interest of American women.”
But he said the agency would require the companies to conduct post-approval
studies involving a total of 80,000 women to continue monitoring the safety of
the implants. He said that information would be collected about rates of
rupture, cancer and autoimmune diseases and effects of the implants on
reproduction. That would enable the agency to evaluate concerns about the
implants in a large number of women.
But Ms. Allina questioned the validity of such studies. “The F.D.A. has no
credibility to assert post-approval studies will be any better,” Ms. Allina
said. “Once again, the F.D.A. is putting the interests of this administration’s
allies, the economic interests of the industry, over public health.”
The agency approved the devices for all women for reconstruction following
breast cancer, trauma, or for developmental disorders affecting the chest. But
the agency said that they would not be available to women under 22 for cosmetic
use.
“We wanted to make sure that breast development had been completed before these
devices had been implanted,” Dr. Schultz said, adding that the agency also did
not have clinical data on younger women. “We concluded that age 22 was the
appropriate age for the lower limit for augmentation.”
Some surgeons, who have participated in studies, will be able to implant the
devices as early as Monday. For others, there will be a wait of up to several
weeks because the F.D.A. is requiring them to participate in a training program
before receiving shipments.
Silicone breast implants were available in the United States as early as the
1960s. Following complaints and lawsuits in the 1970s and 1980s that the devices
ruptured and became hard and painful and that some women developed cancer and
autoimmune diseases, implant makers agreed to take their products off the market
in the United States in 1992, except for treating mastectomy patients and in
some other special cases and only when the patients were enrolled in clinical
studies.
Eventually, thousands of women in this country and elsewhere sued the
manufacturers of breast implants, resulting in class-action settlements by
several companies, including 3M, Baxter, Bristol-Myers and Dow Corning. All got
out of the silicone breast implant business.
In 1999, the Institute of Medicine, an arm of the National Academy of Sciences,
said that while the implants could rupture and become hard and painful, there
was no definitive evidence that they were associated with serious diseases,
including autoimmune disease or cancer.
Dr. Scott L. Spear, the chief of plastic surgery at Georgetown University who
has conducted clinical research for Allergan of Irvine, Calif., said the devices
had been improved.
“The shells themselves are made of different materials, a barrier shell, that is
relatively much more impermeable,” Dr. Spear said. “The shells are thicker than
in ‘91, much thicker than they were in earlier generations. The material inside
is more cohesive, the stuff tends to stick together.”
In Canada, which also withdrew the devices, the implants were cleared for sale
and implantation in October.
Denise Grady contributed reporting.
F.D.A. Will Allow
Breast Implants Made of Silicone, NYT, 18.11.2006,
http://www.nytimes.com/2006/11/18/washington/18breast.html?hp&ex=1163912400&en=1e358875c6245624&ei=5094&partner=homepage
Op-Ed Contributor
Our Great Depression
November 17, 2006
The New York Times
By ANDREW SOLOMON
DEPRESSION is the leading cause of disability worldwide,
according to the World Health Organization. It costs more in treatment and lost
productivity than anything but heart disease. Suicide is the 11th most common
cause of death in the United States, claiming 30,000 lives each year.
Despite medical advances in the last 20 years that have greatly improved our
ability to help those who suffer from depression, we lack an effective system
for administering care. Only a very small percentage of depressives who seek
help receive appropriate treatment for their condition. Research often stalls
short of being translated into useful medicine. Depressives continue to be
stigmatized, which makes their lives even more difficult and lonely. Finally,
many sufferers are left to spiral, unsupported, into despair because their
insurance companies refuse to pay for treatment.
These problems are similar to those cancer patients once faced, and the best way
to address them might be similar as well. We need a network of depression
centers, much like the cancer centers established in the 1970s.
Through the National Cancer Institute, federal funds were dispersed to
interdisciplinary centers like Memorial-Sloan Kettering in New York and M.D.
Anderson in Houston. The idea was to make sure that 80 percent of the American
population lived within 200 miles of such a center.
As this network of institutions took root, the quality of cancer treatment
advanced dramatically. The centers brought researchers and clinicians under one
roof, ensuring that basic science was applied to achieve medical results.
Scientists communicated both within and between centers, so that everyone could
make use of everyone else’s work to accelerate progress.
Following this model, the National Institute of Mental Health should coordinate
and subsidize a national network of depression centers, ideally based at
research universities with good hospitals and departments devoted to the
subject.
The University of Michigan, host to the country’s first national depression
center, which opened its doors last month, has been a pioneer in this regard.
More than 135 experts on depression and bipolar disorder will collaborate there,
about half of them psychiatrists. The center has a large clinical treatment
program and a genetic database that will house samples from tens of thousands of
depressed and bipolar patients. It is sponsoring social and biological research
and pressing for policy initiatives related to mental illness.
Among the thousands of depressed people I have met with, the majority have
sought treatment but feel that they are not getting good care. Many of them have
been prescribed antidepressants by family doctors who lack training in
psychiatry and have conducted only cursory interviews before rendering their
diagnoses. Antidepressants vary in their chemistry and effects; and human brains
vary as much as human minds. To treat the most complicated organ in the body
appropriately demands considerable expertise.
The question I am asked most frequently is how to get better care, and it can be
devilishly hard to answer. Depression centers that could deliver a high standard
of comprehensive care would be a dream come true — not only for millions of
depressives, but also for the research community.
Last winter, the Library of Congress organized a conference where theoreticians
met with mental-health consumer advocates and clinicians. The combination was
unusual and wonderful. Everyone left with fresh ideas. We need formal bodies to
sustain such fruitful intimacy.
Research related to a major disease should not unfold in a purely intellectual
context, nor should consumer advocacy exist solely in a lobbying context, nor
clinical practice exclusively under the shadow of profit-driven pharmaceutical
research. (Full disclosure: my father is the chief executive of a pharmaceutical
company that manufactures antidepressants.)
Before the cancer centers came around, cancer was as taboo as depression is now.
But as antibiotics and vaccines for other illnesses lengthened life expectancy,
cancer became more pervasive and less shameful. Depression, too, is becoming
more widespread and more frequently diagnosed. Depression and bipolar illness
will affect some 20 percent of Americans during their lives, and yet the stigma
endures. People often come up to me after lectures to whisper about their
affliction, as though everyone else in the room weren’t grappling with precisely
the same thing.
It is neither wise nor feasible for a large proportion of the population to be
trying to keep a secret. A national network that helped to medicalize depression
in the public imagination would reduce sufferers’ shame. The very waiting rooms
of depression centers would provide incontrovertible proof of the ubiquity of
the illness and ease the isolation of sufferers. Within the centers, patients
would find themselves the focus of an elite community of insight and support.
Alleviating stigma will also make it harder for insurance companies to deny
treatment. As it is established that these mental illnesses are not character
defects, but instead can be characterized in terms of brain symptoms, the false
distinctions between them and cancer or heart disease will become impossible to
sustain. The fiscal irresponsibility of leaving untreated an illness that causes
enormous loss of productive work years would be clearly demonstrated.
We’ve made stellar progress in treating mental illness since the Prozac
revolution but there is a catastrophic divide between research and practice. We
must come up with a seamless way to support scientific progress and to
administer the treatments we have, in order ultimately to alleviate as much
suffering as possible.
Andrew Solomon, the author of “The Noonday Demon: An Atlas of Depression,”
is on the national advisory board of the University of Michigan Comprehensive
Depression Center.
Our Great
Depression, NYT, 17.11.2006,
http://www.nytimes.com/2006/11/17/opinion/17solomon.html
Stem Cells Help Dogs With Dystrophy
November 16, 2006
By THE ASSOCIATED PRESS
Filed at 4:18 a.m. ET
The New York Times
NEW YORK (AP) -- In promising new research, stem cells
worked remarkably well at easing symptoms of muscular dystrophy in dogs, an
experiment that experts call a significant step toward treating people.
''It's a great breakthrough for all of us working on stem cells for muscular
dystrophy,'' said researcher Johnny Huard of the University of Pittsburgh, who
wasn't involved in the work.
Sharon Hesterlee, vice president of translational research at the Muscular
Dystrophy Association, called the result one of the most exciting she's seen in
her eight years with the organization. Her group helped pay for the work.
She stressed that it's not yet clear whether such a treatment would work in
people, but said she had ''cautious optimism'' about it.
Two dogs that were severely disabled by the disease were able to walk faster and
even jump after the treatments.
The study was published online Wednesday by the journal Nature. It used stem
cells taken from the affected dogs or other dogs, rather than from embryos. For
human use, the idea of using such ''adult'' stem cells from humans would avoid
the controversial method of destroying human embryos to obtain stem cells.
The Nature paper focuses on Duchenne muscular dystrophy, a muscle-wasting
genetic disorder that occurs in about 1 in every 3,500 male births. It's the
most severe and most common childhood form of muscular dystrophy and the
best-known. In theory, the stem cell treatment might also help other muscle
dystrophies or even age-related muscle wasting, Hesterlee said.
Children with the disorder have trouble walking as early as preschool, and
nearly all of them lose their ability to walk between ages 7 and 12. Typically,
they die in their 20s because of weakness in their heart and lung muscles. There
is no known cure.
The dog study was done by Giulio Cossu, director of the stem cell institute at
the San Raffaele Scientific Institute in Milan, Italy, with colleagues there and
elsewhere.
''We do not know whether this will work in patients,'' Cossu said in a telephone
interview. He said he hopes to start a small experiment in children in the next
year or two.
The scientists worked with golden retrievers that suffer a crippling form of
dystrophy very much like the human one. Researchers studied the effect of
repeated injections into the bloodstream of a kind of stem cell extracted from
blood vessel walls.
The best results appeared when the cells were taken from healthy dogs. But Cossu
said scientists should pursue the possibility of genetically manipulating a
patient's own cells and using them instead. That way, patients wouldn't have to
undergo lifelong treatment to avoid rejection of donated cells.
In one of several experiments, three dogs that had not yet shown impairment in
walking were injected five times, a month apart, with cells taken from other
dogs.
One dog completely avoided symptoms and continued to walk well even five months
after both the injections and the anti-rejection therapy were stopped.
A second dog also did well initially but died suddenly of a heart problem after
just two months on the treatment. It's not clear whether the problem had
anything to do with the treatment, or whether the initial good result would have
continued, Cossu said.
The third dog showed partial protection, being able to walk and even run with a
limp, but then progressively lost walking ability within a few days after the
anti-rejection treatment was stopped.
The researchers also treated two dogs that were severely impaired by the
disease. Both gained the ability to move much faster and to jump, and one was
even able to run, although neither could use the hind legs normally.
One of these dogs rapidly lost walking ability when the anti-rejection treatment
was stopped, but the other continued to walk well for five months until
succumbing to pneumonia. That's a common fate for dogs with the genetic
condition because of weakness in breathing muscles.
Cossu said he believed that a human treatment could be directed more at
breathing muscles than it was in the dogs.
The cells helped strengthen muscle by fusing with regenerating muscle fibers and
pumping out a protein that's missing in dogs with the disease.
------
On the Net:
Nature: http://www.nature.com/nature
Muscular Dystrophy Association: http://www.mda.org
Stem Cells Help
Dogs With Dystrophy, NYT, 16.11.2006,
http://www.nytimes.com/aponline/us/AP-Stem-Cells-Dystrophy.html
News Analysis
When Blind Faith in a Medical Fix Is Broken
November 16, 2006
The New York Times
By DENISE GRADY
A blocked artery is not a good thing. Public health
campaigns have drilled that message into the national psyche. Surely, then,
whenever doctors find a closed artery, especially in the heart, they should open
it.
Maybe not. A major study, presented Tuesday at a medical conference in Chicago,
challenged the widespread use of tiny balloons and metal stents in people who
had suffered heart attacks days or weeks before.
Although such treatment can be lifesaving in the early stages of a heart attack,
the study found that opening the artery later did no good at all. It merely
exposed patients to the discomfort, risk and $10,000 expense of an invasive
procedure.
The new report is the latest example of a rigorous experiment turning medical
practice on its head by proving that a widely accepted treatment is not the
great boon it was thought to be (except maybe to the bank accounts of doctors,
drug companies and makers of medical devices).
Ideally, treatments, operations and diagnostic procedures should be thoroughly
tested before they come into routine use. But that is not always the case. Drugs
and medical devices have to be approved by the Food and Drug Administration, but
once they are on the market, doctors can prescribe them in almost any way they
see fit, a practice called off-label use.
Migraine drugs are prescribed for weight loss, and heart pills for stage fright;
nobody is breaking the law. At least one in five drug prescriptions are for
unapproved uses, studies show, with some popular medicines getting more than 90
percent of their use as treatments for which they were never approved. Ideas for
such uses may be suggested to doctors by drug companies.
The approval rules for devices are looser than those for drugs, and while there
is little data measuring unapproved uses of medical devices, there are hints
that off-label use there is even greater. The F.D.A. does not regulate surgery
at all.
Some treatments — like opening a closed artery — appeal so strongly to common
sense that it becomes irresistible to go ahead and use them without waiting for
scientific proof that they are effective. That is especially true if patients
are desperate and have few or no other options.
As the treatments start to catch on, people assume they must work, and it
becomes difficult or impossible to study them in the most definitive way — by
comparing treated patients with an untreated control group. If most people think
a therapy works, who wants to be the control? Doctors may balk at controlled
studies, too, calling it unethical to withhold the treatment from patients in
the control group.
Dr. Judith S. Hochman, a cardiologist at New York University who directed the
recent study on stents, said she encountered exactly that attitude when she was
trying to recruit other researchers for her study: some refused to participate,
saying it was unethical to leave some patients without stents.
But the counterargument is that it is also unethical to subject people to
medicines, operations and invasive tests and treatment without proof that they
are safe and effective.
Medical history is strewn with well-intended treatments that rose and then fell
when someone finally had the backbone to test them, and the scientific method
trumped what doctors thought they knew.
Hormone treatment after menopause, which works for symptoms like hot flashes,
was widely believed to prevent heart disease and urinary incontinence. But
carefully done studies in recent years have shown that hormones can actually
make those conditions worse.
Stomach ulcers were once attributed to emotional stress and too much stomach
acid, and were treated with surgery, acid-blocking drugs and patronizing advice
to calm down. Then, in the 1980s, two doctors who were initially ridiculed for
proposing an outlandish theory proved that most ulcers are caused by bacteria
and can be cured with antibiotics.
For decades, women with early-stage breast cancer were told that mastectomies
offered them the best chance of survival. But in 1985, a large nationwide study
showed that for many, a lumpectomy combined with radiation worked just as well.
“As a nation, we’re not doing ourselves any favors by going after the next new
thing without doing the studies,” said Dr. James N. Weinstein, chairman of
orthopedic surgery at Dartmouth and a researcher at its Center for the
Evaluative Clinical Sciences, which studies how well various medical and
surgical procedures work.
When established treatments turn out to be useless, or worse, harmful, Dr.
Weinstein said, “everybody’s going to lose trust in the system.”
Gardiner Harris contributed reporting.
When Blind Faith
in a Medical Fix Is Broken, NYT, 16.11.2006,
http://www.nytimes.com/2006/11/16/science/16heart.html?hp&ex=1163739600&en=9e3d7e639a5e3a6e&ei=5094&partner=homepage
Success at polls heartens anti-smoking advocates
Posted 11/15/2006 8:50 PM ET
USA Today
By Emily Bazar
Emboldened by victories at the polls last week, an
anti-smoking group said Wednesday that it will push for more state and local
smoking bans around the country.
Among the victories: Voters approved statewide smoking bans
in Arizona, Nevada and Ohio. At the local level, voters in Mankato, Minn., and
Appleton, Wis., quashed attempts to weaken or repeal existing bans.
"Election Day was huge for our movement," says Aaron Doeppers, director of the
Midwest region of the Campaign for Tobacco-Free Kids.
The election "just showed us how far the trend can go," he says.
Doeppers says it created momentum for smoking bans everywhere, not just in
places that have a history of adopting smoke-free laws, such as California.
"We expect to see a huge increase in the number of communities that want to
consider these policies, and we'll support them," he says.
In Appleton, voters were asked to exempt about 60 taverns from the city's
workplace smoking ban, which took effect in July 2005. The bid failed 57% to
43%, city clerk Cindi Hesse says.
It was the second attempt to amend the ban at the ballot box. The first, in
April, also failed.
Mankato voters were asked whether to uphold or repeal their smoking ban, which
took effect in July. Residents voted 69% to 31% to keep the ban, according to
the Blue Earth County Elections Division.
Bars and restaurants that could show they suffered a drop in business since July
are temporarily exempt from the ban. The exemptions expire July 1, 2007.
City manager Pat Hentges says city leaders don't plan to further amend or
otherwise change the ban.
"The argument that the ban was an infringement on business practices and rights
and would have a negative impact on the economy was not generally accepted by
the voters," he says.
Success at polls
heartens anti-smoking advocates, UT, 15.11.2006,
http://www.usatoday.com/news/health/2006-11-15-smoking-ban-polls_x.htm
Troubled Children
What’s Wrong With a Child? Psychiatrists Often Disagree
November 11, 2006
The New York Times
By BENEDICT CAREY
Paul Williams, 13, has had almost as many psychiatric
diagnoses as birthdays.
The first psychiatrist he saw, at age 7, decided after a 20-minute visit that
the boy was suffering from depression.
A grave looking child, quiet and instinctively suspicious of others, he looked
depressed, said his mother, Kasan Williams. Yet it soon became clear that the
boy was too restless, too explosive, to be suffering from chronic depression.
Paul was a gifted reader, curious, independent. But in fourth grade, after a
screaming match with a school counselor, he walked out of the building and
disappeared, riding the F train for most of the night through Brooklyn, alone,
while his family searched frantically.
It was the second time in two years that he had disappeared for the night, and
his mother was determined to find some answers, some guidance.
What followed was a string of office visits with psychologists, social workers
and psychiatrists. Each had an idea about what was wrong, and a specific
diagnosis: “Compulsive tendencies,” one said. “Oppositional defiant disorder,”
another concluded. Others said “pervasive developmental disorder,” or some
combination.
Each diagnosis was accompanied by a different regimen of drug treatments.
By the time the boy turned 11, Ms. Williams said, the medical record had taken
still another turn — to bipolar disorder — and with it a whole new set of drug
prescriptions.
“Basically, they keep throwing things at us,” she said, “and nothing is really
sticking.”
At a time when increasing numbers of children are being treated for psychiatric
problems, naming those problems remains more an art than a science. Doctors
often disagree about what is wrong.
A child’s problems are now routinely given two or more diagnoses at the same
time, like attention deficit and bipolar disorders. And parents of disruptive
children in particular — those who once might have been called delinquents, or
simply “problem children” — say they hear an alphabet soup of labels that seem
to change as often as a child’s shoe size.
The confusion is due in part to the patchwork nature of the health care system,
experts say. Child psychiatrists are in desperately short supply, and family
doctors, pediatricians, psychologists and social workers, each with their own
biases, routinely hand out diagnoses.
But there are also deep uncertainties in the field itself. Psychiatrists have no
blood tests or brain scans to diagnose mental disorders. They have to make
judgments, based on interviews and checklists of symptoms. And unlike most
adults, young children are often unable or unwilling to talk about their
symptoms, leaving doctors to rely on observation and information from parents
and teachers.
Children can develop so fast that what looks like attention deficit disorder in
the fall may look like anxiety or nothing at all in the summer. And the field is
fiercely divided over some fundamental questions, most notably about bipolar
disorder, a disease classically defined by moods that zigzag between periods of
exuberance or increased energy and despair. Some experts say that bipolar
disorder is being overdiagnosed, but others say it is too often missed.
“Psychiatry has made great strides in helping kids manage mental illness,
particularly moderate conditions, but the system of diagnosis is still 200 to
300 years behind other branches of medicine,” said Dr. E. Jane Costello, a
professor of psychiatry and behavioral sciences at Duke University. “On an
individual level, for many parents and families, the experience can be a
disaster; we must say that.”
For these families, Dr. Costello and other experts say, the search for a
diagnosis is best seen as a process of trial and error that may not end with a
definitive answer.
If a family can find some combination of treatments that help a child improve,
she said, “then the diagnosis may not matter much at all.”
A Kaleidoscope of Diagnoses
The most commonly diagnosed mental disorders in younger children include
attention deficit hyperactivity disorder, or A.D.H.D., depression and anxiety,
and oppositional defiant disorder.
All these labels are based primarily on symptom checklists. According to the
American Psychiatric Association’s diagnostic manual, for instance, childhood
problems qualify as oppositional defiant disorder if the child exhibits at least
four of eight behavior patterns, including “often loses temper,” “often argues
with adults,” “is often touchy or easily annoyed by others” and “is often
spiteful or vindictive.”
At least six million American children have difficulties that are diagnosed as
serious mental disorders, according to government surveys — a number that has
tripled since the early 1990s. But there is little convincing evidence that the
rates of illness have increased in the past few decades. Rather, many experts
say it is the frequency of diagnosis that is going up, in part because doctors
are more willing to attribute behavior problems to mental illness, and in part
because the public is more aware of childhood mental disorders.
At the playground, in the gym, standing in line at the grocery store, parents
swap horror stories about diagnoses, medications or special education classes.
Their children are often as fluent in psychiatric jargon as their mothers and
fathers are.
“The change in attitude is enormous,” said Christina Hoven, a psychiatric
epidemiologist at Columbia University. “Not long ago people did all they could
to hide problems like these.” Attention deficit disorder is perhaps the most
straightforward diagnosis. Elementary school teachers are often the ones who
first mention it as a possibility, and soon parents are answering questions from
a standard checklist: Does the child have difficulty sustaining attention,
following instructions, listening, organizing tasks? Does he or she fidget,
squirm, impulsively interrupt, leave the classroom?
These behaviors are so common, particularly in boys, that critics question
whether attention disorder is a label too often given to boys being boys. But
most psychiatrists agree that while many youngsters are labeled unnecessarily,
most children identified with attention problems could benefit from some form of
therapy or extra help.
They are less certain about the children — perhaps a quarter of those seen for
mental problems, some experts estimate — who do not fit any one diagnosis, and
who often go for years before receiving a satisfactory label, if they receive
one at all.
These youngsters collect labels like passport stamps, and an increasing number
end up with the label Paul Williams received: bipolar disorder.
An Illness Under Dispute
Until recently, psychiatrists considered bipolar disorder to be all but
nonexistent in children under 18. Today, it is the fastest growing mood disorder
diagnosed in children, featured on the cover of news magazines and on daytime
talk shows like “The Oprah Winfrey Show.”
The explosion of interest in bipolar disorder came after the approval of several
drugs, called antipsychotics, or major tranquilizers, for the short-term
treatment of mania in adults.
Beginning in the 1990s some researchers began to argue that bipolar disorder was
underdiagnosed in adults. Soon, several child psychiatrists were arguing that
the illness was more common than previously thought in children too.
Some experts who made those arguments had ties to manufacturers of antipsychotic
drugs, financial interests disclosed in professional journals. But the message
struck a chord, particularly with doctors and parents trying to manage difficult
children.
Parents whose children have been given the label tend to adopt the psychiatric
jargon, using terms like “cycling” and “mania” to describe their children’s
behavior. Dozens of them have published books, CDs, or manuals on how to cope
with children who have bipolar disorder.
A recent Yale University analysis of 1.7 million private insurance claims found
that diagnosis rates for bipolar disorder more than doubled among boys ages 7 to
12 from 1995 to 2000, and experts say the rates have only gone up since then.
Katherine Finn, a 14-year-old who lives in Grand Rapids, Mich., said she was
grateful for the growing awareness of the disease. Possessed by feelings of
worthlessness as early as the fourth grade, Katherine said that by the sixth
grade she “threw my sanity out the window.”
She became impulsive, loud, and abrasive, she said, adding, “I would blurt
things out in class, I would moo like a cow, act like a little kid, just say the
most random stuff.”
A psychiatrist promptly diagnosed the problem as bipolar disorder, after
learning that there was a history of the disease on her mother’s side of the
family. Katherine began taking drugs that blunted the extremes in her mood, and
she now is doing well at a new school.
“It hit me like a Mack truck when I heard the diagnosis, but I knew right away
it was correct,” said her mother, Kristen Finn, who is writing a book about her
experience.
Still, many psychiatrists believe that, although childhood bipolar disorder may
be real in families like the Finns, it is being wildly overdiagnosed. One of the
largest continuing surveys of mental illness in children, tracking 4,500
children ages 9 to 13, found no cases of full-blown bipolar disorder and only a
few children with the mild flights of excessive energy that could be considered
nascent bipolar disorder — a small fraction of the 1 percent or so some
psychiatrists say may suffer from the disease.
Moreover, the symptoms diagnosed as bipolar disorder in children often bear
little resemblance to those in adults. Instead, the children’s moods seem to
flip on and off like a stoplight throughout the day, and their upswings often
look to some psychiatrists more like extreme agitation than euphoria.
“The question with these kids is whether what we’re seeing is a form of mania,
or whether it’s extreme anger due to something else,” said Dr. Gregory Fritz,
medical director of the Bradley Hospital, a psychiatric clinic for children in
Providence, R.I.
Dr. Ellen Leibenluft, a research psychiatrist at the National Institute of
Mental Health, argues that children who are receiving a diagnosis of bipolar
disorder fall into two broad groups. The children in one group, a minority, have
mood cycles similar to those of adults with bipolar disorder, complete with
grandiose moods, and a high likelihood of having a family history of the
illness. Those in the other group have severe problems regulating their moods
and little family history, and may have some other psychiatric disorder instead.
“It is a mistake to lump them all together and assume they are all the same,”
Dr. Leibenluft said. “It may be that the disorder has different dimensions and
looks different in different kids.”
For parents with a child who is frantic and possibly dangerous, these
distinctions may be academic. The medications may blunt their child’s extreme
behavior, which may be all the confirmation they need.
For others, though, the uncertainties about childhood bipolar disorder loom
larger. They wonder whether mania really explains what their child is going
through, and if not, what it is that is being treated.
Evelyn Chase of Richmond, Va., said that a neurologist drove home his diagnosis
of bipolar disorder in her 10-year-old son by pulling out “a copy of Time
magazine and slamming the article in front of me.”
Ms. Chase said her son seemed to react most strongly to abrupt changes in the
environment and to certain dyes and chemicals. “I used the bipolar diagnosis for
school and getting services, but I don’t think it covers his behaviors,” she
said.
For Paul Williams, the diagnosis simply feels like a temporary stop. In his
short life, Paul has taken antidepressants like Prozac, antipsychotic drugs used
to treat schizophrenia, sleeping pills and so-called mood stabilizers for
bipolar disorder, in so many combinations that he has become nonchalant about
them.
“Sometimes they help, sometimes they don’t,” he said. “Sometimes they make me
feel like another person, like not normal.”
In recent months, his mother said, Paul seems to have improved: he visibly tries
to control himself when he is upset and usually succeeds. He is an eager Mets
fan who loves reading Harry Potter and the Goosebumps series. He gets out and
plays baseball and football, like any 13-year-old boy.
But he has grown tired of telling his story to doctors, and neither he nor his
mother expect that bipolar disorder will be the last diagnosis they hear.
In Search of Clarity
The specialists who manage children’s psychiatric disorders are trying to bring
more standards and clarity to the field. Harvard researchers are completing the
most comprehensive nationwide survey of mental illness in minors and hope to
publish a report next year. And a recent issue of the journal Child and
Adolescent Psychology was entirely devoted to the subject of basing diagnoses in
hard evidence.
Given the controversies, one of the articles concludes, “we acknowledge that
tackling the issue may be tantamount to taking on a 900-pound gorilla while
still wrestling with a very large alligator.”
Dr. Darrel Regier of the American Psychiatric Association, who is coordinating
work on the next edition of the association’s diagnostic manual for mental
disorders, due out in 2011, said that researchers would focus on drawing
distinctions among several childhood disorders, including bipolar disorder and
attention deficit disorder.
“We wouldn’t disagree that criteria for these disorders currently overlap to
some degree,” Dr. Regier wrote in an e-mail message, “and that a significant
amount of research is under way to disentangle the disorders in order to support
more specific treatment indications.”
Until that happens, parents with very difficult children are left to read the
often conflicting signals given by doctors and other mental health
professionals. If they are lucky, they may find a specialist who listens
carefully and has the sensitivity to understand their child and their family.
In dozens of interviews, parents of troubled children said that they had
searched for months and sometimes years to find the right therapist.
“The point is that not everything is A.D.H.D., not everything is bipolar, and it
doesn’t happen like you see in the movies,” said Dr. Carolyn King, who treats
children in community clinics around Detroit, and has a private practice in the
nearby suburb of Grosse Pointe Farms.
“Kids often have very subtle symptoms they can mask for short periods of time,”
Dr. King said, “and the most important thing is to observe them closely, and get
a complete history, starting from birth and straight through every single
developmental milestone.”
She added, “A speech delay can look like anxiety,” an obsessive private ritual
like mania.
Or struggling children, in the end, may look only like themselves, with a unique
combination of behaviors that defy any single label. Camille Evans, a mother in
Brooklyn whose son’s illness was tagged with a half-dozen different diagnoses in
the last several years, said she concluded, after seeing several psychiatrists,
that the boy’s silences and learning difficulties were best understood as a mild
form of autism.
“That’s the diagnosis that I think fits him best, and I’ve just about heard them
all,” Ms. Evans said.
The label is not perfect, she said, but it is more specific than “developmental
delay” — one diagnosis they heard — and does not prime him for aggressive
treatment with drugs like attention deficit disorder or bipolar disorder would.
He has not responded well to the drugs he has tried.
“Most important for me,” Ms. Evans said, “the diagnosis gives him access to
other things, like speech therapy, occupational therapy and attention from a
neurologist. And for now it seems to be moving him in the right direction.”
What’s Wrong With
a Child? Psychiatrists Often Disagree, NYT, 11.11.2006,
http://www.nytimes.com/2006/11/11/health/psychology/11kids.html
Medicaid Wants Citizenship Proof for Infant Care
November 3, 2006
The New York Times
By ROBERT PEAR
WASHINGTON, Nov. 2 — Under a new federal policy, children
born in the United States to illegal immigrants with low incomes will no longer
be automatically entitled to health insurance through Medicaid, Bush
administration officials said Thursday.
Doctors and hospitals said the policy change would make it more difficult for
such infants, who are United States citizens, to obtain health care needed in
the first year of life.
Illegal immigrants are generally barred from Medicaid but can get coverage for
treatment of emergency medical conditions, including labor and delivery.
In the past, once a woman received emergency care under Medicaid for the birth
of a baby, the child was deemed eligible for coverage as well, and states had to
cover the children for one year from the date of birth.
Under the new policy, an application must be filed for the child, and the
parents must provide documents to prove the child’s citizenship.
The documentation requirements took effect in July, but some states have been
slow to enforce them, and many doctors are only now becoming aware of the
effects on newborns.
Obtaining a birth certificate can take weeks in some states, doctors said.
Moreover, they said, illegal immigrant parents may be reluctant to go to a state
welfare office to file applications because they fear contact with government
agencies that could report their presence to immigration authorities.
Administration officials said the change was necessary under their reading of a
new law, the Deficit Reduction Act, signed by President Bush in February. The
law did not mention newborns, but generally tightened documentation requirements
because some lawmakers were concerned that immigrants were fraudulently claiming
United States citizenship to get Medicaid.
Marilyn E. Wilson, a spokeswoman for the Tennessee Medicaid program, said: “The
federal government told us we have no latitude. All states must change their
policies and practices. We will not be able to cover any services for the
newborn until a Medicaid application is filed. That could be days, weeks or
months after the child is born.”
About four million babies are born in the United States each year, and Medicaid
pays for more than one-third of all births. The number involving illegal
immigrant parents is unknown but is likely to be in the tens of thousands,
health experts said.
Doctors and hospitals denounced the policy change and denied that it was
required by the new law. Dr. Jay E. Berkelhamer, president of the American
Academy of Pediatrics, said the policy “punishes babies who, according to the
Constitution, are citizens because they were born here.”
Dr. Martin C. Michaels, a pediatrician in Dalton, Ga., said that continuous
coverage in the first year of life was important because “newborns need care
right from the start.”
“Some Americans may want to grant amnesty to undocumented immigrants, and others
may want to send them home,” Dr. Michaels said. “But the children who are born
here had no say in that debate.”
Under a 1984 law, infants born to pregnant women on Medicaid are in most cases
deemed eligible for Medicaid for one year.
In an interview on Thursday, Leslie V. Norwalk, acting administrator of the
Centers for Medicare and Medicaid Services, said the new policy “reflects what
the new law says in terms of eligibility.”
“When emergency Medicaid pays for a birth,” Ms. Norwalk said, “the child is not
automatically deemed eligible. But the child could apply and could qualify for
Medicaid because of the family’s poverty status. If anyone knows about a child
being denied care, we want to know about it. Please step up and tell us.”
Under federal law, hospitals generally have to examine and treat patients who
need emergency care, regardless of their ability to pay. So the new policy is
most likely to affect access to other types of care, including preventive
services and treatment for infections and chronic conditions, doctors said.
Representative Charlie Norwood, Republican of Georgia, was a principal architect
of the new law.
“Charlie’s intent was that every person receiving Medicaid needs to provide
documentation,” said John E. Stone, a spokesman for Mr. Norwood, who is a
dentist and has been active on health care issues. “With newborns, there should
be no problem. All you have to do is provide a birth certificate or hospital
records verifying birth.”
But Dr. Berkelhamer disagreed. Even when the children are eligible for Medicaid,
he said, illegal immigrants may be afraid to apply because of “the threat of
deportation.”
The new policy “will cost the health care system more in the long run,” Dr.
Berkelhamer added, because children of illegal immigrants may go without
immunizations, preventive care and treatments needed in the first year of life.
Doctors, children’s hospitals and advocacy groups have been urging states to
preserve the old policy on Medicaid eligibility for children born to illegal
immigrants.
Sara Rosenbaum, a professor of health law at George Washington University, said:
“The new policy reflects a tortured reading of the new law and is contrary to
the language of the 1984 statute, which Congress did not change. The whole
purpose of the earlier law, passed with bipartisan support, was to make sure
that a baby would not have a single day’s break in coverage from the date of
birth through the first year of life.”
California has objected to the new policy. S. Kimberly Belshé, secretary of the
California Health and Human Services Agency, said: “By virtue of being born in
the United States, a child is a U.S. citizen. What more proof does the federal
government need?”
Medicaid Wants
Citizenship Proof for Infant Care, NYT, 3.11.2006,
http://www.nytimes.com/2006/11/03/washington/03medicaid.html
Op-Ed Contributor
The Memory Hole
November 3, 2006
The New York Times
By DAVID SHENK
ONE hundred years ago today, a 42-year-old German
psychiatrist and neuropathologist named Alois Alzheimer shocked colleagues with
his description of one woman’s autopsied brain.
The woman was named Auguste Deter. Five years earlier, her husband had admitted
her to Alzheimer’s psychiatric hospital in Frankfurt with a disturbing set of
symptoms: memory trouble, aphasia (loss of the ability to use words), confusion,
bursts of anger and paranoia. She had become a danger to herself in the kitchen
and needed constant care.
Alzheimer found his new patient sitting on a bed with a helpless expression.
“What is your name?” he asked.
“Auguste,” she replied.
“Last name?”
“Auguste.”
“What is your husband’s name?”
“Auguste, I think.”
“How long have you been here?”
(She seems to be trying to remember, he wrote in his notes.)
“Three weeks.”
It was her second day in the hospital. “I have lost myself,” she told her
doctor. Over the next four and a half years, she grew increasingly disoriented,
delusional and incoherent. She would scream for hours on end. Eventually,
Auguste Deter became bedridden, incontinent and largely immobile, and then, in
April 1906, at age 55, she died.
What was this strange disease that would take an otherwise healthy middle-aged
woman and slowly — very slowly, as measured against most disease models — peel
away, layer by layer, her ability to remember, to communicate her thoughts and
finally to understand the world around her?
It looked like senile dementia, the sharp unraveling of memory and mind that
had, for more than 5,000 years, been accepted by doctors and philosophers as a
routine consequence of aging. But she was too young for senile dementia.
Alzheimer was able to look inside her brain for answers, thanks to a whirl of
European innovation. Ernst Leitz and Carl Zeiss had just invented the first
distortion-free microscopes. Franz Nissl had revolutionized tissue-staining,
making various cell constituents stand out, opening up what was characterized as
“a new era” of the study of brain cells and tissues.
(The Nissl method, by the way, is still in use. Nissl, a friend and close
collaborator of Alzheimer, became a medical school legend with his instructions
on how to time the staining process: Take the brain out, he advised. Put it on
the desk. Spit on the floor. When the spit is dry, put the brain in alcohol.)
With Auguste Deter’s brain tissue fixed, frozen, sliced, stained and pressed
between two thin pieces of glass, Alzheimer put down his habitual cigar, removed
his pince-nez, and peered into his state-of-the-art Zeiss microscope. Then, at a
magnification of several hundred times, he finally saw her disease.
It looked like measles, or chicken pox, of the brain. The cortex was speckled
with crusty brown clumps — we now call them plaques — too many to count. They
varied in size, shape and texture and seemed to be a hodgepodge of granules and
short, crooked threads, as if they were sticky magnets for microscopic trash.
The plaques were nestled between the neurons, blocking their communication with
one another. They were so prominent that Alzheimer could see them without any
stain at all, but they showed up best in a blend of magenta red, indigo carmine
and picric acid.
A different stain revealed what Alzheimer called “a tangled bundle of fibrils” —
weedy, menacing strands of rope bundled densely together. These tangles grew
inside the nerve cells, strangling them.
Auguste Deter had not lost herself. Rather, her “self” was taken from her.
On Nov. 3, 1906, Alzheimer presented his findings at the 37th meeting of
South-West German Psychiatrists with a paper titled, “Regarding a Curious
Disease of the Cortex.” What he did not realize was that these very same plaques
and tangles were not just responsible for this rare, middle-aged dementia, but
also for the majority of cases of senile dementia.
Nor could he have foreseen that with the significant rise in longevity over the
20th century, cases of Alzheimer’s disease would skyrocket into the millions.
Paradoxically, we have created a civilization of such health and longevity that
a disease that was once rare now threatens us all.
There’s no good way to die, but some are far worse — and far costlier — than
others. The plodding progression of Alzheimer’s devastates not only the patient
but also a wide circle of family and friends forced to witness and participate
in the long decline. The disease costs a fortune in medical and nursing fees and
lost wages; a conservative estimate is that the current five million cases in
the United States add up to more than $100 billion annually.
If that sounds like a lot of money, keep in mind that the baby boomers have not
started turning 65 yet. By the middle of this century, 15 million Americans
could have Alzheimer’s — about 100 million people worldwide — and national costs
could reach $1 trillion, threatening to bankrupt our entire health care system.
This is a disease that, if left on course, will greatly affect our economy, our
politics and our communities.
The good news is that scientists now understand much about how the disease
ravages the brain, and have many good ideas about how to stop it. The bad news
is that Alzheimer’s research is expensive, slow and, even in the face of this
growing epidemic, underfinanced.
The political will to find a cure has long been hampered by the stigma and lack
of understanding that surrounds this disease. But we must do what it takes to
cure Alzheimer’s before it saps our economy and steals another generation.
David Shenk is the author of “The Forgetting: Alzheimer’s, Portrait of an
Epidemic.”
The Memory Hole,
NYT, 3.11.2006,
http://www.nytimes.com/2006/11/03/opinion/03shenk.html
|