|
History > 2006 > USA > Health (III)
F.D.A. Says Bayer Failed
to Reveal Drug
Risk Study
September 30, 2006
The New York Times
By GARDINER HARRIS
WASHINGTON, Sept. 29 — Bayer A.G., the German
pharmaceutical giant, failed to reveal to federal drug officials the results of
a large study suggesting that a widely used heart-surgery medicine might
increase the risks of death and stroke, the Food and Drug Administration
announced Friday.
Bayer scientists even appeared at a public meeting called by the F.D.A. on Sept.
21 to discuss the possibility that the drug, Trasylol, might have serious risks.
But they did not mention the study or its worrisome results.
In a highly unusual move, the food and drug agency released a public health
advisory saying it had learned of the study’s existence only on Wednesday.
Preliminary results of the study demonstrate “that use of Trasylol may increase
the chance for death, serious kidney damage, congestive heart failure and
strokes,” the advisory said.
Nevertheless, the agency did not change its advice about whether patients should
be given the drug. Instead, it restated previous warnings that Trasylol’s use
should be limited to patients in whom the risks of blood loss outweighed the
drug’s risks.
The disclosure comes exactly two years after Merck announced it was withdrawing
its arthritis drug, Vioxx, after a study showed that it doubled the risks of
heart attacks. Since then, members of Congress and even top scientific advisers
have concluded that the F.D.A. lacks the regulatory authority and the money
needed to detect and protect against drug dangers.
Drug companies have also been sharply criticized for failing to make public the
results of some human trials of their drugs that suggest that the drugs are
either ineffective or dangerous. Some lawmakers have proposed legislation that
would require that nearly all human drug trials must be announced and their
results disclosed publicly.
A top F.D.A. official said the agency learned of the Trasylol study on Wednesday
only after a getting a tip from a researcher involved in it. The official
insisted on anonymity because of the sensitive nature of the information.
In a written statement, Bayer said “that it mistakenly did not inform” the
F.D.A. of the study and added, “This data was not shared immediately with the
agency because it was preliminary in nature.”
Staci Gouveia, a Bayer spokeswoman, said the company nonetheless stood behind
the safety of Trasylol, which has become one of Bayer’s fastest sellers. Sales
last year were $200 million and were expected to nearly triple this year.
Several members of the advisory committee that met last week said they were
shocked that Bayer failed to inform them of the study.
“For them not to mention that it was under way, that it was being analyzed or
that results were available is appalling and will do significant harm to their
reputation for transparency,” said Dr. John Teerlink, an associate professor of
medicine at the University of California, San Francisco, and a member of the
advisory committee.
Steven Findlay, a health care analyst at Consumers Union and another committee
member, said the agency needed to investigate whether Bayer knowingly withheld
the information from the advisory committee.
“The safety of this drug is called into further question now,” Mr. Findlay said.
Doctors give Trasylol to patients before surgery to reduce the risks of blood
loss. It can also reduce the need for transfusions in patients undergoing heart
bypass surgery. Trasylol, also known as aprotinin, has been on the market for 13
years.
But two recent studies suggested that the drug might have serious risks. One of
the articles, published in January in The New England Journal of Medicine, found
that the drug increases the risks of kidney failure, heart attack and stroke.
The study concluded that halting the drug’s use would prevent 10,000 to 11,000
cases of kidney failure a year and save more than $1 billion a year in dialysis
costs, as well as nearly $250 million spent on the drug itself.
There are other, cheaper drugs that can be used in Trasylol’s place.
Still, the advisory panel concluded that Trasylol’s risks were worth taking in
some patients. Dr. Teerlink said that despite the results of the new study, that
might still be true.
Bayer’s study was performed by a contract research organization. But Bayer did
not inform the F.D.A. that the study was being done, even though that is routine
practice.
It examined hospital records of 67,000 patients, 30,000 of whom received
Trasylol. The rest got other drugs. It concluded that the patients given
Trasylol were at greater risk.
Such studies, however, are fraught with statistical and other problems. Patients
given Trasylol may have been sicker than those given other drugs. Their worse
outcomes would be explained not by problems with Trasylol but by their own
illness.
Susan Bro, an F.D.A. spokeswoman, said it was evaluating the new study and would
decide soon whether the results merit changing the agency’s advice about use of
the drug.
“It is regrettable that the F.D.A. advisory board did not have the benefit of a
frank scientific dialogue based on the totality of available data,” she said.
Senator Charles E. Grassley, Republican of Iowa and chairman of the Senate
Finance Committee and a longtime critic of the F.D.A., said Bayer’s behavior
proved that the agency was largely toothless.
“The remedy is mandatory reporting of all clinical trials and real teeth for the
F.D.A. to do its job in holding drug companies accountable,” Mr. Grassley said.
F.D.A. Says Bayer Failed to Reveal Drug Risk Study, NYT, 30.9.2006,
http://www.nytimes.com/2006/09/30/health/30fda.html
Economix
The Choice:
A Longer Life or More Stuff
September 27, 2006
The New York Times
By DAVID LEONHARDT
The most authoritative report on the cost of
health insurance came out yesterday, and it’s sure to cause some new outrage.
The average cost of a family insurance plan that Americans get through their
jobs has risen another 7.7 percent this year, to $11,500, according to the
Kaiser Family Foundation. In only seven years, the cost has doubled, while
incomes and company revenue, which pay for health insurance, haven’t risen
nearly as much.
These spiraling costs — a phrase that has virtually become a prefix for the
words “health care” — are slowly creating a crisis. Many executives have decided
that they cannot afford to keep insuring their workers, and the portion of
Americans without coverage has jumped 23 percent since 1987.
An industry that once defined the American economy, meanwhile, is sinking in
large measure because of the cost of caring for its workers and retirees. For
every vehicle that General Motors sells, fully $1,500 of the purchase price goes
to pay for medical care. “We must all do more to cut costs,” G.M.’s chief
executive, Rick Wagoner, said on Capitol Hill this summer while testifying about
health care.
Mr. Wagoner’s argument has become the accepted wisdom about the crisis: the
solution lies in restraining costs. Yet it’s wrong. Living in a society that
spends a lot of money on medical care creates real problems, but it also has
something in common with getting old. It’s better than the alternative.
To understand why, it helps to look back to a time when Americans didn’t worry
much about health care costs. In 1950, the country spent less than $100 a year —
or $500 in today’s dollars — on the average person’s medical care, compared with
almost $6,000 now, notes David M. Cutler, an economist who wrote a wonderful
little book in 2004 titled, “Your Money or Your Life.”
Most families in the 1950’s paid their medical bills with ease, but they also
didn’t expect much in return. After a century of basic health improvements like
indoor plumbing and penicillin, many experts thought that human beings were
approaching the limits of longevity. “Modern medicine has little to offer for
the prevention or treatment of chronic and degenerative diseases,” the biologist
René Dubos wrote in the 1960’s.
But then doctors figured out that high blood pressure and high cholesterol
caused heart attacks, and they developed new treatments. Oncologists learned how
to attack leukemia, enabling most children who receive a diagnosis of it today
to triumph over a disease that was almost inevitably fatal a half-century ago.
In the last few years, orphan drugs that combat rare diseases and medical
devices like the implantable defibrillator have extended lives. Human longevity
still hasn’t hit the wall that was feared 50 years ago.
Instead, a baby born in the United States this year will live to age 78 on
average, a decade longer than the average baby born in 1950. People who have
already made it to their 40’s can now expect to reach age 80. These gains are
probably bigger than the ones the British experienced in the entire millennium
leading up to 1800. If you think about this as the return on the investments in
medicine, the payoff has been fabulous: Would you prefer spending an extra
$5,500 on health care every year — or losing 10 years off your lifespan?
Yet we often imagine that the costs and benefits are unrelated, that we can
somehow have 2006 health care at 1950 (or even 1999) prices. We think of health
care as if it were gasoline, a product whose price and quality have nothing to
do with each other.
There is no question that the American medical system does suffer from a lot of
waste, be it insurance industry bureaucracy or expensive procedures that haven’t
been proven effective. But the No. 1 cause of the cost increases is still the
one you can see at the hospital and in your medicine cabinet — defibrillators,
chemotherapy, cholesterol drugs, neonatal care and other treatments that are
both expensive and effective.
Not even most forms of preventive care, like keeping diabetes under control,
usually save money, despite what many people think. The care itself has some
costs, and, more important, patients then live longer than they otherwise would
have and rack up medical bills. “When I make this point, people accuse me of
wanting people to die earlier. But it’s exactly the opposite,” Dr. Jay
Bhattacharya, a researcher at Stanford Medical School, told me. “If these
expenditures are keeping people alive, it’s money well spent.”
As Dr. Mark R. Chassin of the Mount Sinai School of Medicine in New York says,
“You almost always spend money to gain health.” Of course, the opposite is also
true: the best way to reduce health care spending is to reduce health care
itself.
Which is exactly what we’re starting to do. The growing number of families
without health insurance are, in effect, families who have been kicked off the
country’s health care rolls. Many will go without available treatment, will get
sicker than they need to get — and will thereby save the rest of us money. They
are what now passes for a solution to the health care mess.
The current situation is indeed unsustainable, a point that the conventional
wisdom has right. The cost of health insurance can’t keep doubling every seven
years, and wasteful spending — the brand-name drugs that are no better than
generics, the treatments that haven’t been proved to extend lives or improve
health — does need to be reined in.
But far too much of the discussion has been centered on this narrow idea.
Somehow, going to the mall to buy clothes has come to be seen as a vaguely
patriotic way to keep the economy humming, and taking out a risky mortgage is
considered to be an investment in one’s future. But medical care? That’s just a
cost.
It’s easy to be against high costs, and it will no doubt be hard to come up with
a broad health care solution. But the way to start is by acknowledging that an
affluent society should devote an ever-growing share of its resources to the
health of its citizens. “We have enough of the basics in life,” Mr. Cutler, the
economist and author, points out. “What we really want are the time and the
quality of life to enjoy them.”
The
Choice: A Longer Life or More Stuff, NYT, 28.9.2006,
http://www.nytimes.com/2006/09/27/business/27leonhardt.html
Life Expectancy Data
September 27, 2006
The New York Times
By DAVID LEONHARDT
For most of human history, the average
lifespan was considerably less than 50 years. It began to rise markedly in the
19th century, hitting 49 in the United States in 1900, and then took off in the
20th century.
“Life expectancy increased only very slowly for two millennia,” said Richard
Suzman, director of the Social and Behavioral Research Program at the National
Institute on Aging, “and then almost doubled since 1800.”
Today, the average baby born in this country will live to 78. The average 35
year old will live to about 80. And the average 65 year old will live past 83.
For detailed data going back to 1900, click here and then go to Table 11, which
appears on page 30 of the document.
Life
Expectancy Data, NYT, 28.9.2006,
http://www.nytimes.com/2006/09/27/business/27leonhardt_sidebar.html
Related >
http://www.cdc.gov/nchs/data/nvsr/nvsr54/nvsr54_14.pdf
Best cardiac care
found in wealthiest
areas,
GNS analysis shows
Updated 9/27/2006 10:42 PM ET
By Robert Benincasa and Jennifer Brooks,
Gannett News Service
USA Today
WASHINGTON — Heart patients in wealthier
communities have a better chance of getting recommended treatments at their
local hospitals than patients in lower-income areas.
A Gannett News Service analysis of how
frequently U.S. hospitals gave recommended treatments to those suffering from
heart attacks and heart failure found that the best-performing hospitals are
much more concentrated in the nation's higher-income counties.
In low-income counties, top-performing hospitals are scarce. In counties ranking
in the lowest 20% for median household income, only 5% of the hospitals were in
the highest of five performance rankings for heart attack patients. Nearly half
of them fell into the lowest category.
In high-income counties, a quarter of the hospitals were top performers.
"Hospitals serving poor populations have very limited resources, and they simply
cannot provide the high-quality care delivered in your mainstream hospitals,"
said Dr. Ernest Moy, who leads the team that writes the federal government's
National health care Disparities Report.
Moy said the GNS findings are consistent with other disparities researchers have
documented in American health care. "In almost everything we track, there's a
very large socioeconomic effect, and it's as large or larger than the racial or
ethnic effect," he said.
The most recent report from Moy's group looked at 13 key measures of health care
quality and found poor people got lower quality care than high-income people on
11 of them.
Using data provided by hospitals to the federal Centers for Medicare and
Medicaid Services and covering the period of October 2004 through September
2005, GNS rated the nation's hospitals on heart care. The ratings show how often
they gave standard treatments to heart attack and heart failure patients who
were supposed to get them.
Treatments included giving heart attack patients aspirin, which reduces the
tendency of blood to clot, and beta-blocker drugs, which reduce the stress on
the heart.
Based on how hospitals ranked in comparison to other hospitals, GNS awarded them
from one to five stars for heart attack and heart failure treatments, both
nationally and within their states. Hospitals with very few heart patients
weren't included. Some 3,100 U.S. hospitals, or about three-quarters of those in
a federal government database, received GNS heart attack ratings, and 3,600
received heart failure ratings.
The heart treatment data come from a government and industry consortium aimed at
measuring and improving hospital quality. The group chose heart measures among
its first indicators because heart attacks and heart failure are common, serious
problems that are relatively easy to track. Hospitals around the country have
improved on the indicators since the consortium began systematically collecting
and publishing the data.
In 2006, an estimated 1.2 million Americans will have a heart attack, and heart
disease will kill more U.S. adults than any other single cause.
Other indicators of quality
In addition to the household income of a hospital's home county, there were
other powerful indicators associated with hospital performance. Among them:
- Medical school affiliation. This made a dramatic difference in following
guidelines, particularly for heart attack patients. Among larger teaching
hospitals, those with 500 beds or more, more than a third earned top ratings for
heart attack treatments. Very few of those facilities — 3% — were in the lowest
performance category.
Advocates for teaching hospitals say the variety of specialized personnel and
educational emphasis adds up to more attention to patients and more cutting edge
science.
- Urban vs. rural. Only about one in nine rural hospitals fell into the
top-scoring category for treating heart attack patients. More than a third were
in the lowest category. Urban hospitals were unlikely to be in the lowest
category, with about 13%. For heart failure patients, nearly a third of rural
hospitals fell into the lowest performance category.
The reason is likely linked to the disadvantages rural hospitals may face when
trying to improve quality: limited resources, small staffs, low patient volume
and lagging information technology. Rural hospitals also face shortages of
specialty doctors and other providers, according to the National Advisory
Committee on Rural Health and Human Services.
- Geography. On average, hospitals in Southern states provided standard
treatments for heart attack patients about 87% of the time. In other regions,
the average was more than 90%. Western states lagged in standard treatments for
heart failure patients, with a 67% average rate, below a national average of
72%.
- Accreditation. Nearly half of the hospitals without a current accreditation
from the Joint Commission on Accreditation of health care Organizations were
bottom-tier performers for heart failure patients. For treating heart attacks,
40% were bottom-tier performers.
- Ownership type. Government-owned and for-profit hospitals generally performed
poorly in comparison to hospitals with other ownership arrangements.
Church-owned facilities performed well for both types of heart patients studied.
Public hospitals tend to get less revenue from their patients, many of whom are
uninsured. And with typically small operating margins, they may find it harder
to fund quality improvement programs and computer systems.
"They're always going to be stretched thin," said Dr. Benjamin Chu, a former
head of New York City's public hospital corporation who is credited with
improving quality there.
Making an informed choice
Kim Miller, a 48-year-old single mom and businesswoman, thought through her
hospital choice, despite being in the throes of an attack.
Doubled over with chest pains and nausea — symptoms of a heart attack — Miller
had two hospitals to choose from in her hometown of Poughkeepsie, N.Y., one
night in December 2003, as she telephoned a friend for a ride to the emergency
room.
One of the hospitals, Vassar Brothers, is the city's heart center and performs
cardiac surgery.
"I knew that Vassar has the cardiac center," said Miller, who directed her
friend to drive her to that hospital, rather than the emergency room at St.
Francis Hospital.
In 2004-05, Vassar Brothers performed above the state and national medians for
providing correct care to heart attack patients, and earned five stars in the
GNS analysis for landing in the top category nationally and within New York
state.
The other hospital, St. Francis, performed below the medians and earned one
star.
Miller is happy about her choice and the treatment she received at Vassar
Brothers. But St. Francis President and CEO Bob Savage said it wouldn't have
made a difference if she had chosen his hospital instead.
"All hospitals really can treat an acute myocardial infarction, a heart attack.
The difference in Vassar Brothers and St. Francis at this point is they do have
the cardiac catheterization lab, and cardiac catheterization is the ultimate
diagnostic tool."
With catheterization, doctors thread a thin tube into the heart through an
intravenous line, and use it to check heart functions.
Savage said the hospital's scores were low because of a charting problem.
"Basically we were not doing a good job of documenting exclusions," he said.
"There really are reasons why some people should not receive aspirin or beta
blockers."
The next batch of numbers on heart treatments, Savage said, will be much
improved, reflecting better documentation as well as an improvement in care.
Still, says disparities researcher Moy, "The average consumer should look at how
their local hospital is doing and consider going to another hospital if their
hospital is in the bottom (group)."
Best
cardiac care found in wealthiest areas, GNS analysis shows, UT, 27.9.2006,
http://www.usatoday.com/news/health/2006-09-27-hospitals-analysis-main_x.htm
The Last Holdout
Reconsiders a Program to
Curb H.I.V.
September 25, 2006
The New York Times
By RICHARD G. JONES
CAMDEN, N.J., Sept. 21 — On most days, the
fringe workers in this city’s stunningly vibrant drug trade shout and
gesticulate from street corners like hot dog vendors at a ballpark, hawking
hypodermic needles they claim are clean.
“Works for sale! Works for sale!”
But the shouting stopped at one corner recently after one of those dealers died
of a drug overdose. For his loyal customers, it was a disaster, however briefly.
“It was a drought of works,” said Maria Lugo, 26, who said that she injects
heroin eight times a day. “People were picking up old needles off the street.
They didn’t care. They just wanted to get off.”
That mix of apathy and addiction has led to what health officials say is a
public health crisis in New Jersey, where state figures show that more than 4 in
10 cases of H.I.V. infection result from injecting illegal drugs with
contaminated needles. Even so, New Jersey remains the one state that prohibits
the distribution of hypodermic needles in government-sanctioned programs.
That may soon change. In a reversal that even supporters acknowledge would have
been unexpected as recently as six months ago, lawmakers may well pass a bill
soon to allow needle exchanges in New Jersey.
The legislation, to be considered this week, appears finally to have enough
support to win passage, 14 years after it was first introduced, and Gov. Jon S.
Corzine has said he would sign it.
To supporters, the bill is long overdue, and the Legislature’s refusal to
approve it has contributed to the deaths of an untold number of drug users.
To opponents, including State Senator Thomas H. Kean Jr., the Republican nominee
for the United States Senate, the measure would mean nothing less than a
government endorsement of illegal drug use.
One of the bill’s most vocal critics, State Senator Ronald L. Rice, a former
Newark police officer who represents a district in Essex County, has compared
legal needle exchanges to the notorious Tuskegee Syphilis Study, an experiment
in Alabama from 1932 until 1972 in which proper treatment was withheld from
African-Americans infected with venereal disease.
Mr. Rice has argued that exchange programs contribute to a cycle of social and
economic inertia for minorities and the poor.
A better use of state money, he says, would be to create more programs providing
drug education and treatment.
“Those who want a syringe access program are saying that it’s O.K. for drug
users to continue using drugs and kill themselves because it’s cheaper for the
government,” he said.
It was Mr. Rice, a Democrat, who negotiated an alliance with the Republican
minority in the Senate to prevent the needle exchange bill from getting out of
committee as recently as last spring.
But last Monday, committee members reached a compromise that would allow pilot
needle exchange programs in six cities. The program would be re-evaluated in
five years under the bill, which would also provide $10 million for drug
treatment programs.
State Senator Nia H. Gill, a Democrat from Essex County who sponsored the
original legislation, cited statistics that show New Jersey is the state with
the third-highest rate of H.I.V. infection among children, and the highest rate
among women.
Nearly 33,000 residents of New Jersey have AIDS, according to state health
officials, and, in a statement, Ms. Gill noted that almost as many — about
30,000 — have died of the disease.
“If there were any other cause of hundreds of deaths of children, thousands of
deaths of women and the orphaning of tens of thousands of children — our
Legislature would act on an emergency basis to reduce that cause,” she said.
To those who see the consequences of the drug trade in neighborhoods like South
Camden, an exchange program cannot come too soon.
Several times a week, Jose Quann and Johnny Brown, workers with the Camden Area
Health Education Center, drive a modified recreational vehicle through some of
the bleakest parts of the city to do street-level prevention and education
concerning H.I.V. and AIDS.
They hand out free condoms to prostitutes. They give intravenous-drug users
bleach kits to sterilize syringes. And they offer health services like free
blood pressure screenings and new oral H.I.V. tests that yield results in 20
minutes.
Bouncing over the uneven asphalt along Broadway in South Camden the other day,
Mr. Brown, a burly 51-year-old, said that he did not believe free needles would
encourage drug abuse.
“When they take part in needle exchange, it means they’re starting to take an
interest in their health,” Mr. Brown said. “That’s the first step.”
Mr. Quann, 46, agreed. “It’s a win-win for everybody,” he said. “Right now,
they’re disposing of these things in our back alleys, in our playground where
our kids are getting pricked.”
Although the six cities in the proposed pilot program have not been chosen, both
men hope that Camden, whose 75,000 residents, according to city figures, include
an estimated 800 people with H.I.V. or AIDS, will be one of them.
So did a half-dozen visitors to their bus.
One of them, a 30-year-old prostitute who said her first name was Summer,
sneered at the idea that providing free needles would promote drug use.
“Users are going to use anyway,” she said. “But you’d have less people infected
with H.I.V., hepatitis.”
The
Last Holdout Reconsiders a Program to Curb H.I.V., NYT, 25.9.2006,
http://www.nytimes.com/2006/09/25/nyregion/25needles.html
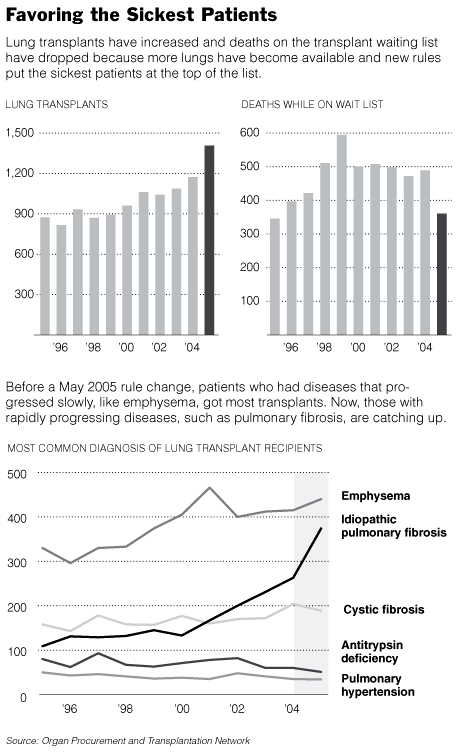
Lung Patients See a New Era of Transplants
NYT 24.9.2006
http://www.nytimes.com/2006/09/24/health/24lung.html
Lung Patients
See a New
Era of Transplants
September 24, 2006
The New York Times
By DENISE GRADY
A quiet revolution in the world of lung
transplants is saving the lives of people who, just two years ago, would have
died on the waiting list.
In the past 16 months, waits have shortened, lists have shrunk, and the number
of lung transplants has gone up. Further improvements are expected this year.
The changes have all but erased the need for transplants from live donors —
desperate, last-ditch operations requiring two donors per patient, usually
relatives and friends who risk major surgery in hopes of rescuing a loved one
whose time is running out.
“It’s almost as if it’s a whole new day for lung transplantation,” said Dr.
Cynthia Herrington, a surgeon at the University of Minnesota Medical Center,
Fairview, in Minneapolis. “It’s amazing.”
Nationwide, it is too soon to tell what the impact of the transplant changes
will be.
“Are we actually improving overall survival?” asked Dr. Selim Arcasoy, the
medical program director for lung transplantation at New York-Presbyterian
Hospital/Columbia University. “Or are we transplanting sicker people who don’t
last as long?”
Transplants are given to people whose lungs fail because of emphysema, cystic
fibrosis or other, less common diseases. Since demand exceeds supply, patients
must join regional waiting lists that are part of a national network.
Recent changes have revitalized lung transplantation. Starting in May 2005, new
rules nationwide put patients who needed transplants most at the top of the list
— people who would soon die without a transplant, but who had a good chance of
surviving after one.
Previously, lungs went to whoever had been waiting longest, even if another
patient needed them more. The waiting time was often two years or more, so there
was little hope for people with lung diseases that came on suddenly or
progressed rapidly.
Another major change is that more lungs from cadavers have become available, for
two reasons: more people are becoming organ donors, and doctors have figured out
ways to salvage lungs that previously would have been considered unusable. The
new methods use drugs, respirator settings and other techniques to prevent
damage to the lungs and keep their tiny air sacs open in brain-dead patients.
In the past, lungs could be retrieved from only about 15 percent of organ
donors, but at some centers the rates have risen to 40 percent. Dr. Herrington
said that in Minnesota, the number of lungs retrieved went to 97 from 25 in a
single year.
“Good organs 5 or 10 years ago were probably being buried” because doctors did
not know how to save them, said Dr. Kenneth R. McCurry, director of heart and
lung transplantation at the University of Pittsburgh.
The number of lung transplants has risen to 1,405 in 2005, 248 more than the
year before. Fewer people are dying on the waiting list: 360 in 2005, down from
488 in 2004.
The new rules made the difference between life and death for Hannah Olson, 20, a
college student from Waukon, Iowa, with cystic fibrosis, a genetic disease that
affects the lungs and digestive system. Ms. Olson was well enough to start
college in 2004, but by January 2006, she was on the transplant list. Her
status, though, was not yet listed as “active,” because her condition seemed
stable and she needed to gain weight.
But in mid-February her lungs gave out. Unable to breathe, Ms. Olson was put on
a respirator, and her waiting list status changed to active. Without a
transplant, she probably would have died within days. Her desperate condition
translated into a numerical score that shot her to the top of the list. Twelve
hours later, lungs became available, and she received her transplant at the
Fairview center in Minneapolis, one day after being put on the active list.
“I’d probably be gone if the list was the way it was before,” Ms. Olson said.
Her surgeon, Dr. Herrington, agreed, saying, “In the old system she would not
have even been listed, because she would have had years to wait.”
Ms. Olson is back in college now, hoping to earn a degree in social work. “I’d
kind of like to work with transplant patients,” she said.
Lungs have always been “the bad stepchild” of organ transplants — harder to get,
harder to transplant, more prone to rejection and complications than other
organs, said Dr. Scott Palmer, the medical director of Duke University’s lung
transplant program. Lung transplants were not consistently successful until the
mid-1980’s, lagging far behind those of kidneys, livers and hearts. From the
start, lungs have been offered first to whoever had spent the most time on the
waiting list, in the donor’s geographic region.
Changes in the system came about partly because of a 1998 federal regulation
requiring that all organ transplants go to patients with the greatest medical
need. The intention was to even out waiting times around the country and
decrease deaths on the waiting list. Changes have been gradual.
Livers and hearts are already allocated according to patients’ needs. Kidneys
still depend on waiting time, but the rules may change to factor in patients’
odds for survival, according to Annie Moore, a spokeswoman for the United
Network for Organ Sharing, or UNOS, the nonprofit organization that manages the
transplant network in the United States.
For lungs, figuring out how to measure medical need and rank patients with
different diseases took time.
“Our concern was that if we used just severity of illness, we might waste a lot
of lungs on patients who were so sick they were unlikely to survive anyway,”
said Dr. Thomas Egan, a cardiothoracic surgeon at the University of North
Carolina, Chapel Hill, who led a UNOS panel that spent several years developing
new rules for lung allocation.
The panel studied medical records to figure out which patients were most or
least likely to survive after a transplant, and worked that into the scoring
system. As a result, lungs are now the only organs with transplant rules that
consider the recipient’s survival odds.
Almost immediately, the new system cut the waiting list in half. Because waiting
time no longer mattered, people who had been listed early in their illness just
to hold a place in line dropped in rank or were deleted (unless they needed a
transplant right away) but could rejoin the list later if they became sicker.
Overnight, some patients who had waited for years to reach the top of the list
suddenly found themselves at the bottom, or even crossed off. Nobody was
grandfathered in.
“We tried our best to educate and communicate, but many felt they had been
cheated,” Dr. McCurry said. But at his center in Pittsburgh there were no deaths
among those who lost their places in line, he said, adding that many still
received transplants.
Those who remained on the list needed transplants soon. As a result, it became
much easier to find recipients quickly, which was a huge improvement, because
once an organ donor is brain dead, organs start to deteriorate. The lungs are
especially fragile.
In the past, transplant coordinators might have spent hours calling hospitals,
only to hear again and again that the patient at the top of the lung list was
not sick enough for a transplant. Meanwhile the clock would be ticking; patients
would have been found who needed the heart, kidneys and liver; and surgeons
would be standing by, ready to remove them. Doctors say some lungs were probably
wasted because recipients simply could not be found fast enough.
“Placement is easier now,” Dr. Egan said. “It takes four or five calls. It used
to be 16.”
The new system has also changed the types of patients who receive the most
transplants. Before, a majority had emphysema, a lung disease nearly always
brought on by smoking. They received transplants because the disease moves
slowly and they could wait, outlasting patients — often younger ones — with
other lung diseases.
“People with pulmonary fibrosis or pulmonary hypertension can be diagnosed and
go downhill very, very rapidly,” said Dr. G. Alexander Patterson, a surgeon at
Washington University in St. Louis, which has one of the country’s largest lung
transplant programs, with about 55 to 60 adult patients and 25 to 30 children a
year.
Pulmonary fibrosis causes extensive lung scarring, and its cause is often
unknown. Patients can die within a year of the diagnosis. But patients with
emphysema can often live for a long time. As a result, Dr. Patterson said, many
people thought the old system gave an unfair advantage to emphysema patients.
On the waiting list, 5 to 10 percent with emphysema died each year, compared
with 30 to 40 percent among those with cystic fibrosis or pulmonary fibrosis.
“It was an ethical dilemma,” Dr. Patterson said, adding that some doctors were
troubled to see so many transplants go to people with emphysema, which is caused
by smoking, whereas “others have disease they didn’t produce.”
Now, at most centers, more patients with pulmonary fibrosis are getting
transplants.
Dr. Jonathan B. Orens, medical director of the lung transplant program at Johns
Hopkins, said that in the past year, more than half the 28 recipients there were
people who, under the old system, would have died on the waiting list.
Dr. Orens said he and his colleagues had just performed a preliminary analysis
of the nationwide data on the first patients treated under the new system, and
found that so far, one-year survival rates appeared to have dropped, to about 70
percent, from about 80 percent over all.
“At first blush, that may seem like a bad thing,” he said. But, he said, it may
mean that the new system is doing exactly what was intended: giving transplants
to the patients who need them most, rather than to people who do not need them
yet.
Past survival rates might have been higher because the recipients were
healthier. But a transplant might not have prolonged those patients’ lives
because they might have survived just as long without it. Sicker patients may
not live as long as others after a transplant but may still live longer than
they would have without it.
“You get only a limited longevity from a lung transplant,” Dr. Orens said. “In
the old system, the average survival was a little under five years.”
Earlier studies suggested that although people with emphysema might have felt
better after transplants, they did not necessarily gain time, whereas those with
pulmonary fibrosis or cystic fibrosis did. That finding means that the emphysema
patients may have been getting transplants too soon, Dr. Orens said.
“This is important,” he said. “If you transplant patients prematurely, you may
shorten their lives.”
Slightly lower survival rates under the new system, he said, “may be the best we
can do with lung transplants when patients are this sick.”
The rates may still represent a net benefit, he said, “compared to shortening
the lives of patients who did not quite need the transplant.”
As more data comes in, the rules may need to be adjusted, Dr. Orens said. “We’re
trying to capture just the right patients at just the right time.”
Lung
Patients See a New Era of Transplants, NYT, 24.9.2006,
http://www.nytimes.com/2006/09/24/health/24lung.html
As Children Suffer,
Parents Agonize Over
Spinach
September 24, 2006
The New York Times
By MONICA DAVEY
MILWAUKEE, Sept. 22 — The hardest moment came
when Elaine and Dennis Krause’s 9-year-son, who had stoically undergone kidney
dialysis, blood transfusions and drug treatments that made him hallucinate that
spiders were all around him, quietly asked his parents whether he was going to
die.
“What do you say when your child asks you, am I going to die?” asked Elaine
Krause, who has spent nearly all of this month beside her son’s bed at a
hospital here, watching his skinny, lanky body do battle with E. coli. “I told
him, ‘These people are trying to help you, and you are getting good care.’ But
the truth is, I couldn’t answer him directly. We didn’t know.”
By this weekend, a national outbreak of E. coli linked to fresh spinach grown in
California had sickened 170 people in 25 states and killed at least one here in
Wisconsin, where more people have grown ill than in any other state. The
authorities in Idaho and Maryland were investigating the deaths of two others,
including a toddler whose parents said they gave him a fruit smoothie with
spinach days earlier, trying to determine whether their deaths, too, were linked
to the outbreak.
But beyond the raw statistics were individual stories of a sudden, mysterious,
life-threatening illness, one that struck most often in the homes of those who
viewed themselves as more health conscious than many other Americans.
In many cases, it crept up with frightening force after what had seemed a
harmless, even healthful meal — a spinach salad with walnuts, a sandwich layered
with spinach or, as for the Krause family, a baked, boneless, skinless piece of
chicken on a small bed of spinach. Then what had seemed a simple bout of
diarrhea in the morning often led to a harrowing, bloody race to the emergency
room by midnight.
And around the country, some families still wait by bedsides, wondering which
foods they could ever again feel safe giving their children, what the government
or the spinach industry could have done to protect them, and, most of all,
whether their loved ones will ever fully recover.
“Here you think you’re feeding your child a great, healthy meal,” Dennis Krause
said sadly. “But here I was, poisoning him.”
Not all E. coli is harmful to humans, but certain strains produce toxins that
kill cells in the gut and in the blood vessels, leading to abdominal cramps and
watery to bloody diarrhea, said Dr. John Flaherty, associate chief of the
division of infectious disease at the Feinberg School of Medicine at
Northwestern University.
One area where the toxins can wreak particular havoc is in the kidneys; when it
happens, Dr. Flaherty said, the inflamed cells lining the blood vessels get
“roughed up,” causing red blood cells to break open as they pass by and jam
blood flow to the kidneys.
“In the course of one week, he went from this healthy, lively little boy to a
boy in a hospital bed fighting for his life,” said Anne Grintjes, of Brookfield,
Wis., whose 7-year-old son developed a dangerous form of kidney failure linked
to E. coli, called hemolytic uremic syndrome. “He turned yellow and gray,
literally. It was shocking and terrifying and unbelievable to watch.”
Of the 171 cases that have been confirmed as part of the spinach outbreak, more
than half required hospitalization, and about 16 percent developed the dangerous
hemolytic uremic syndrome.
Doctors say they cannot be certain what long-term effects the syndrome may have;
it varies from person to person. Many patients recover entirely, they say, while
others may suffer from chronic kidney problems and other health risks.
Although the youngest children and the oldest adults are considered most
vulnerable to getting sick from the bacteria and to struggling to recover from
its effects, more than 70 percent of those who grew ill in this outbreak were
somewhere in between: older than 4 and younger than 65.
At 27, Gwyn Wellborn of Salem, Ore., described a sudden onslaught of slicing,
stabbing abdominal pain and bloody diarrhea in late August, days after eating
baby spinach at work, thinking she was being especially virtuous. At first,
doctors sent her home, Ms. Wellborn said, with a painkiller and a diagnosis of
food poisoning. Hours later, though, the pain became so excruciating and the
bleeding so constant, she said, that her husband rushed her back to a hospital.
From there, she quickly deteriorated. Her kidneys began failing. “At one point,
the doctors told my family there was nothing they could do, that that was it,”
she recalled. But after three transfusions and several weeks in a hospital, Ms.
Wellborn went home.
Hers is one of at least four lawsuits filed in courts around the country by
William D. Marler, a Seattle lawyer, in connection with the outbreak. Ms.
Wellborn said she would not consider eating spinach anytime soon. “Not in a
million years,” she said.
Marion Graff, 77, a former bank clerk and widow from Manitowoc, Wis., is the
nation’s only confirmed fatality in the outbreak, but deaths in Maryland and
Idaho are also considered “suspect” cases, federal authorities said on Friday.
Kyle Allgood, 2, of Chubbuck, Idaho, died at a Utah hospital from cardiac arrest
after contracting hemolytic uremic syndrome, his family said, a case Idaho
authorities said was “probably connected” to the tainted spinach, although
laboratory tests have been inconclusive. Kyle’s mother, Robyn Allgood, said she
had mixed vegetables into blended fruit and juice smoothies, as a way to get
Kyle and her two daughters to eat vegetables, and had given him such a drink on
Sept. 12.
Three days later, she said, Kyle had flu-like symptoms, including severe
diarrhea.
On Wednesday, despite specialized treatment at a hospital in Salt Lake City,
Kyle died, hours after his father, Jeff, said he had urged the boy “to fight for
me.”
Relatives tearfully recalled Kyle as generous and good-natured though
mischievous — a boy known as the “stuntman” for his antics, which inspired fear
and awe in adults.
He liked to twirl his hair into knots, dance wildly to the music of Jack Johnson
and give lingering kisses to his aunts, the family said. He was also impish; at
Primary Children’s Medical Center in Salt Lake City, Kyle was pinching his
nurses and ripping at his IV’s until near his final moments, they said.
In Maryland, an elderly woman died of E. coli on Sept. 13 after eating fresh
spinach, state health officials confirmed, but because of a lack of DNA
specimens from the victim, officials have yet to determine whether it was the
same strain of E. coli that infected others who ate spinach. Family members
identified the woman as June E. Dunning, 86, of Hagerstown.
Warren Swartz, Ms. Dunning’s son-in-law, said she had eaten steamed spinach from
a package on Aug. 30 and Sept. 1 and baby spinach from a bag on Aug. 30. She had
signs of E. coli, including rectal bleeding and bloody diarrhea, on Sept. 1 and
went to the hospital the next day.
For the Krause family, of McFarland, Wis., the illness began a few days after a
family dinner on Aug. 24. First, their 15-year-old daughter grew ill. Then their
9-year-old, who was healthy enough that he had a perfect attendance record last
year in third grade, grew even more ill, and has suffered debilitating effects,
including acute renal failure, fluid buildup near the heart, raised blood
pressure, soaring blood sugar.
In a hospital cafeteria here, miles from their home, the Krauses at first could
only bring themselves to eat peanut butter and jelly sandwiches. Everything else
— lunch meats , salads, everything — scared them.
“For me, there’s anger and paranoia and fear for others — for the safety of food
we get that’s supposed to be monitored,” Ms. Krause, 47, said. “I don’t know
what to trust. Should we grow it all ourselves?”
On Friday, their strawberry-blond boy lay weakly in his hospital bed, surrounded
by balloons and cards from the school where he says he fears falling behind his
class. He had physical therapy, then occupational therapy to begin rebuilding
his body. He told his father he only wanted to be well, to somehow rewind to the
day before the spinach.
A few days ago, his body seemed to turn a corner and doctors removed him from
the dialysis machine, a crucial step toward recovery. His parents have not asked
when he might be well enough to go home; they said it was too early to look
ahead.
“I keep thinking we will at some point go home and it’ll all be like it was,”
said Mr. Krause, 52. “But we don’t know yet what the long-term effects are. He
will be monitored probably for the rest of his life. It’ll never be the same.”
Libby Sander contributed reporting from Chicago; Martin Stolz from
Bountiful, Utah; and Gary Gately from Baltimore.
As
Children Suffer, Parents Agonize Over Spinach, NYT, 24.9.2006,
http://www.nytimes.com/2006/09/24/us/24spinach.html
Study Condemns
F.D.A.’s Handling of Drug
Safety
September 23, 2006
The New York Times
By GARDINER HARRIS
WASHINGTON, Sept. 22 — The nation’s system for
ensuring the safety of medicines needs major changes, advertising of new drugs
should be restricted, and consumers should be wary of drugs that have only
recently been approved, according to a long-anticipated study of drug safety.
The report by the Institute of Medicine, part of the National Academy of
Sciences, is likely to intensify a debate about the safety of the nation’s drug
supply and the adequacy of the government’s oversight. The debate heated up in
September 2004 when Merck withdrew its popular arthritis drug Vioxx after
studies showed that it doubled the risks of heart attacks.
Several senators have already proposed significant changes, some of which the
report seems to endorse.
The report’s conclusions are often damning. It describes the Food and Drug
Administration as rife with internal squabbles and hobbled by underfinancing,
poor management and outdated regulations.
“Every organization has its share of dysfunctions, unhappy staff members and
internal disputes,” the report said. But panel members said that they were
deeply concerned about the agency’s “organizational health” and its ability to
ensure the safety of the nation’s drug supply.
The report made these recommendations, most of which would require Congressional
authorization:
¶Newly approved drugs should display a black triangle on their labels for two
years to warn consumers that their safety is more uncertain than that of older
drugs.
¶Drug advertisements should be restricted during this initial period.
¶The F.D.A. should be given the authority to issue fines, injunctions and
withdrawals when drug makers fail — as they often do — to complete required
safety studies.
¶The F.D.A. should thoroughly review the safety of drugs at least once every
five years.
¶The F.D.A. commissioner should be appointed to a six-year term.
¶Drug makers should be required to post publicly the results of nearly all human
drug trials.
In a telephone conference with reporters on Friday, top F.D.A. officials struck
an awkward balance between thanking the institute for its work and defending
their own leadership. They said they needed to study the report before deciding
which of its recommendations to endorse.
“While considerable work has been done over the past two years to improve our
approach to drug safety, work still needs to be done,” said Dr. Andrew C. von
Eschenbach, the acting commissioner of the agency and the nominee for
commissioner.
An internal e-mail message sent Friday to agency staff members by Dr. Sandra L.
Kweder, deputy director of the Office of New Drugs, was blunter, bemoaning the
report’s criticism of what it described as the agency’s dysfunctional culture.
“It is a long, inflammatory section of the report that will certainly generate
the most public attention and hit our people hard,” Dr. Kweder wrote, according
to a copy provided to The New York Times.
Agency critics were elated.
“The new report validates what the watchdog community has been saying for the
last two years,” said Senator Charles E. Grassley, Republican of Iowa, who as
chairman of the Senate Finance Committee has overseen investigations into drug
safety problems. “Problems are systemic, and solutions must reflect a new
mind-set by the agency leadership.”
The drug industry, through its trade organization, reacted warily. “Though there
is always room for improvements, it would be a mistake to accept the notion that
the F.D.A. drug safety system is seriously flawed,” said Caroline Loew, senior
vice president of the Pharmaceutical Research and Manufacturers of America.
The Institute of Medicine is a nonprofit organization created by Congress to
advise the federal government on health issues. The report was issued by the
Committee on the Assessment of the United States Drug Safety System, led by
Sheila P. Burke, deputy secretary and chief operating officer of the Smithsonian
Institution.
The report described fierce disagreements between those who approve drugs and
those who study their effects after approval, disputes that repeated F.D.A.
efforts have not resolved. Indeed, managers’ failure to address such
disagreements competently “has played an important role in damaging the
credibility” of the agency, it said.
Critics of the food and drug agency have long been divided into two warring
camps. Some say the agency fails to approve life-saving medicines quickly
enough, while others say that it is so intent on rapid approvals that it fails
to ensure the safety of the drugs.
The institute’s report champions the latter view by calling for greater caution.
It suggests that one of the agency’s biggest problems is a deal struck in 1992
between Congress and the drug industry in which drug makers agreed to pay
millions in fees to speed reviews. This deal has increased pressures on drug
reviewers to act quickly, and it has limited “the ability of reviewers to
examine safety signals as thoroughly as they might like,” the report said.
“Some also have serious concerns that the regulator has been ‘captured’ by
industry it regulates, that the agency is less willing to use the regulatory
authority at its disposal,” the report said, criticizing the agency’s regulatory
tools as “all-or-nothing.”
“The agency needs a more nuanced set of tools to signal uncertainties, to reduce
advertising that drives rapid uptake of new drugs, or to compel additional
studies in the actual patient populations who take the drug after its approval,”
it said.
The pharmaceutical industry is likely to fight at least some of the proposals,
said Charlie Cook, a Washington political analyst.
“One should never underestimate the influence of the drug industry,” Mr. Cook
said. “But I would think that at least the outlines of many of these
recommendations would have a decent chance of getting through Congress.”
Senators Michael B. Enzi, Republican of Wyoming and chairman of the Health,
Education, Labor and Pensions Committee, and Edward M. Kennedy of Massachusetts,
the ranking Democrat on the committee, have jointly proposed a bill that would
undertake at least some of the changes advocated by the report.
Another bill, sponsored by Senator Grassley and Senator Christopher J. Dodd,
Democrat of Connecticut, offers similar proposals.
There is little chance that Congress will act on any of these proposals before
next year, when it must reauthorize the 1992 financing deal with the drug
industry. Negotiations between the drug industry and agency about the parameters
of that deal are already under way.
Despite its fierce criticisms, the report may bolster the confirmation prospects
of Dr. von Eschenbach. A Senate committee approved his nomination on Wednesday,
but two Republican senators have vowed to block it.
Over the past 10 years, no commissioner has served more than two years, though
the term is open-ended. The report deplored this “lack of stable leadership.”
“Without stable leadership strongly and visibly committed to drug safety, all
other efforts to improve the effectiveness of the agency or position it
effectively for the future will be seriously, if not fatally, compromised,” the
report states.
It recommends that the commissioner be nominated for a six-year term, but such a
change may not solve the problem of early exits. President Bush has nominated
two past commissioners. The first left for another job within the
administration; the second left amid accusations of financial improprieties.
The report recommends that Michael O. Leavitt, the secretary of health and human
services, appoint an independent board to advise the commissioner “to implement
and sustain the changes necessary to transform” the agency’s culture.
It rejects suggestions by Mr. Grassley and others that the F.D.A. create a
center for drug safety to monitor drugs after approval.
“Achieving a balanced approach to the assessment of risks and benefits would be
greatly complicated, or even compromised, if two separate organizations were
working in isolation from one another,” the report concludes.
The F.D.A. asked the Institute of Medicine to review its drug safety system
shortly after the Vioxx withdrawal in 2004, and the agency has agreed to pay $3
million for the study.
Study
Condemns F.D.A.’s Handling of Drug Safety, NYT, 23.9.2006,
http://www.nytimes.com/2006/09/23/health/policy/23fda.html
U.S. Urges
H.I.V. Tests for Adults and
Teenagers
September 22, 2006
The New York Times
By DONALD G. McNEIL Jr.
In a major shift of policy, the federal
government recommended yesterday that all teenagers and most adults have H.I.V.
tests as part of routine medical care because too many Americans infected with
the AIDS virus don’t know it.
The recommendation, by the Centers for Disease Control and Prevention, urges
testing at least once for everyone aged 13 to 64 and annual tests for those with
high-risk behavior.
The proposal is a sharp break from the early days of the AIDS epidemic, when the
stigma of the disease and the fear of social ostracism caused many people to
avoid being tested.
That led to heated debate about whether positive test results could be shared by
medical and governmental authorities in their effort to contain the epidemic by
reaching out to partners of those who might be infected.
Under the agency’s plan, which states can adopt or modify if they choose,
patients would be advised they were being tested, but the tests would be
voluntary.
So that the tests could be easily administered, however, the agency urged the
removal of two major barriers that some states now have: separate signed consent
forms and lengthy counseling before each test.
That would require new laws in some states, however, which could take years
because some civil liberties groups and lobbyists for people with AIDS oppose
the changes.
Many doctors are expected to welcome the changes.
“These recommendations are important for early diagnosis and to reduce the
stigma still associated with H.I.V. testing,” said Dr. Nancy Nielsen, a board
member of the American Medical Association, which endorsed the new guidelines.
Dr. Julie Gerberding, the disease control agency’s director and a doctor who
treated some of the first San Francisco AIDS patients in 1981, said: “Our
traditional approaches have not been successful. People who don’t know their own
H.I.V. status account for 50 to 70 percent of all new infections. If they knew,
they would take steps to protect themselves and their partners.”
The new guidelines, if adopted, would move the agency toward its “ultimate
goals,” which Dr. Gerberding described as: no more H.I.V.-infected children, no
one living for years without antiretroviral treatment and, eventually, no more
new cases of the disease.
About 40,000 Americans are newly infected each year, a number that has been
remaining steady. In contrast to the early days of the epidemic, which struck
gay men the hardest, many of those now infected are black or Hispanic, are
teenagers and were infected by heterosexual sex. The agency estimates that
250,000 Americans, a quarter of those with the disease, do not know they are
infected.
Moreover, 42 percent of those who find out they are infected are tested only
because they are already seriously ill — which means they have been infected for
up to 10 years and may have been passing the infection on all that time, Dr.
Gerberding said.
The American Academy of H.I.V. Medicine, a group for AIDS specialists, gave a
qualified endorsement of the guidelines, agreeing with the need for more testing
but arguing that they gave counseling short shrift.
“Counseling just naturally goes with testing, as diet does to exercise,” said
Dr. Jeff Schouten, the academy’s chairman.
Some civil liberties organizations and those representing people with AIDS,
while favoring more testing, have lobbied against removing signed consent forms
or pretest counseling for fear that such changes will make testing less
voluntary.
Some states, including New York, have laws requiring such counseling and consent
forms. They were passed in the early days of the AIDS epidemic, when having the
virus amounted to a death sentence, the disease’s stigma often led to denial of
jobs or housing, and testing was done primarily to protect the blood supply.
Dr. Thomas R. Frieden, New York City’s health commissioner, said yesterday that
he “absolutely” agreed with the new guidelines and had been lobbying the state
Legislature for a law incorporating them.
A bill he supported was introduced late last year, Dr. Frieden said, but
opponents kept it from coming up for a vote.
“I am optimistic that it will make it through this year,” he said.
Rose A. Saxe, a staff lawyer with the AIDS Project of the American Civil
Liberties Union, said her group opposed the recommendation because it would
remove the requirement for signed consent forms and pretest counseling. In
settings like emergency rooms where doctors are strapped for time, Ms. Saxe
said, “we’re concerned that what the C.D.C. calls routine testing will become
mandatory testing.”
Patients, particularly teenagers, she said, “will be tested without an
opportunity for understanding the magnitude of having a positive result.”
David Ernesto Munar, associate director of the AIDS Foundation of Chicago and a
board member of the National Association of People With AIDS, said he favored
more testing and faster counseling to encourage it.
“But our fear,” Dr. Munar said, “is that on the ground, the rush to get more
blood samples is going to railroad right over any consent.”
Illinois, like New York, requires written consent before a test, he said.
Disease control agency experts deny that their guidelines would encourage such
problems.
They oppose mandatory testing, secret testing or testing without informing
patients, at least orally, that such a test will be done. They suggest that
whatever general consent for routine medical care a state law requires include
consent for H.I.V. testing.
They also want anyone who tests positive to be counseled that AIDS is a serious
disease and taught where to get treatment and how to keep from infecting others.
As an example of success in a related program, Dr. Timothy Mastro, acting
director of the agency’s AIDS prevention division, pointed to the agency’s
guidelines to prevent infection of newborns.
The guidelines say that all pregnant women should be tested unless they refuse
and that oral consent is acceptable. They also recommend tests again in late
pregnancy for women who inject drugs, have sex with many men, have sex for money
or live in neighborhoods where AIDS is common.
The number of babies born infected dropped to fewer than 240 a year now from
1,650 in 1991, Dr. Mastro said.
Laws for prisoners, which Dr. Mastro described as “a tricky area,” might also
need revision. In some states, testing is mandatory for all prisoners. In New
York, it is voluntary.
Health officials in other states appeared to welcome the new guidelines. Steve
Huard, spokesman for the Hillsborough County Health Department, which includes
Tampa, Fla., said: “We strongly believe in universal H.I.V. testing through
anonymous and confidential testing. With the recommendations, it would be more
widespread. It would go out to private physicians and we should see infection
rates going down.”
Some states with few AIDS patients, like Wyoming, may be reluctant to adopt the
guidelines on the ground that routine H.I.V. tests would be unnecessarily
burdensome for doctors and patients.
To compensate for that, the guidelines suggest that routine tests might not be
required in areas where fewer than 1 in 1,000 people test positive. But health
care practitioners are not very good at guessing what rate will be found among
their patients, said Dr. Bernard Branson, the C.D.C.’s associate director for
laboratory diagnostics, so there should first be a period of routine testing.
The wholesale cost is about $1 for each test run in batches and about $8 for
rapid tests done individually. Each positive test would require a second
confirmation test and then counseling, which would raise the cost to about $80,
Dr. Branson said.
That is far cheaper than many other routine screening tests like colonoscopies
or mammograms, and Dr. Branson said most such tests were paid for by insurers
because it was usually cheaper to treat diseases when they were caught early.
U.S.
Urges H.I.V. Tests for Adults and Teenagers, NYT, 22.9.2006,
http://www.nytimes.com/2006/09/22/health/22hiv.html
Spinach Pulled From Stores Across U.S.
September 17, 2006
By THE ASSOCIATED PRESS
Filed at 4:45 a.m. ET
The New York Times
LOS ANGELES (AP) -- Shoppers changed their
buying habits Saturday as spinach was pulled from grocery store shelves because
of the outbreak of E. coli bacteria that had killed one person and sickened more
than 100 others.
The U.S. Food and Drug Administration warned consumers not to eat fresh spinach
and Natural Selection Foods LLC recalled its packaged spinach throughout the
United States, Canada and Mexico. The move came as a precaution after federal
health officials said some of those hospitalized reported eating brands of
prepackaged spinach distributed by the company.
The officials stressed that the bacteria had not been isolated in products sold
by the holding company, based in San Juan Bautista, Calif., and known for
Earthbound Farm and other brands. As the investigation continues, other brands
may be implicated, officials said.
At a Safeway grocery in San Francisco's Potrero Hill neighborhood, many of
bagged produce shelves were empty Saturday. Anna Cairns said she had to settle
for bags of iceberg green lettuce and Caesar salad, instead of her normal salad
mix, which contained spinach.
''I have a bag of spinach in my refrigerator I need to throw away,'' said
Cairns, 59, of San Francisco.
Marina Zecevic, 49, of West Los Angeles, shopping at a Trader Joe's, said she
made the mistake of serving creamed spinach to her kids the day the story broke.
''My sons started accusing me of premeditated murder,'' she said.
She felt the contamination issue was overblown.
''The minute we get the all clear, the spinach is back on the table,'' she said.
The spinach, grown in California, could have been contaminated in the field or
during processing, according to the Centers for Disease Control and Prevention.
About 74 percent of the fresh market spinach grown in the U.S. comes from
California, according to the California Farm Bureau Federation. There have been
previous bacterial contamination outbreaks linked to spinach and lettuce grown
in the state.
Wisconsin accounted for nearly a third of the 102 reported illnesses, including
the lone death, a 77-year-old woman who died of kidney failure.
Other states reporting cases were California, Connecticut, Idaho, Indiana,
Kentucky, Maine, Michigan, Minnesota, New Mexico, Nevada, New York, Ohio,
Oregon, Pennsylvania, Utah, Virginia, Washington and Wyoming, according to the
CDC.
''We are very, very upset about this. What we do is produce food that we want to
be healthy and safe for consumers, so this is a tragedy for us,'' Natural
Selection spokeswoman Samantha Cabaluna said.
The FDA advised consumers not to eat fresh spinach or fresh spinach-containing
products until further notice. Some restaurants and retailers may be taking
spinach out of bags before selling it, so consumers shouldn't buy it at all, the
FDA said.
Boiling contaminated spinach can kill the bacteria but washing won't eliminate
it, the CDC warned.
At a Stop and Shop supermarket in Meriden, Conn., Michelle Bookey said she
frequently buys spinach for salads for her dieting husband but plans to cook it
from now on.
''It worries me. I don't even want to buy lettuce,'' said Bookey, 36.
Earthbound Farm, which claims it pioneered the retail market in pre-washed,
bagged salads in 1986, says its spinach and other products are in 74 percent of
U.S. grocery stores.
It also sells spinach to restaurants and other establishments that serve food.
The National Restaurant Association said members were pulling spinach from their
menus.
The recall earned the praise of Tom Stenzel, president and chief executive
officer of the United Fresh Produce Association.
''The FDA investigation and the voluntary action taken by Natural Selection
Foods LLC help narrow concern about any continuing risk, and begins to ensure
that product that may be potentially contaminated is removed completely from the
food supply,'' Stenzel said in a statement.
A Seattle law firm said it planned to add Natural Selection Foods on Monday to
federal lawsuits previously filed in Wisconsin and Oregon that named other
spinach producers.
------
Associated Press writers Andrew Bridges in Washington, Justin M. Norton in San
Francisco, Ryan J. Foley in Madison, Wis., and Shelley Wong of Hartford, Conn.,
contributed to this story.
------
On the Net:
Center for Food Safety and Applied Nutrition:
http://www.cfsan.fda.gov/
Spinach Pulled From Stores Across U.S., NYT, 17.9.2006,
http://www.nytimes.com/aponline/us/AP-Tainted-Spinach.html
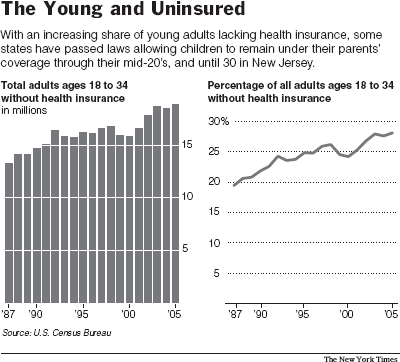
For Insurance, Adult Children Ride Piggyback
NYT 17.9.2006
http://www.nytimes.com/2006/09/17/us/17insure.html
For Insurance,
Adult
Children Ride Piggyback
September 17, 2006
The New York Times
By JENNIFER 8. LEE
Not only are children moving back home after
college and asking Mom and Dad for monthly subsidies, but in a growing number of
states children can now stay on their parents’ health insurance plans well into
their 20’s.
Call it another example of adultescence. With 18- to 34-year-olds the fastest
growing group of uninsured, states are extending the time that children can be a
dependent for insurance purposes.
In New Jersey, which this year enacted the highest age limit, children can
“piggyback” until they turn 30, as long as they live in the state and don’t have
their own children.
The trend stems from a concern that a healthy — and profitable — segment of the
population is dropping out of the insurance pool. About half of all states have
studied such proposals, and at least nine have passed laws, eight of them since
2003 and three just this year, according to the National Conference of State
Legislatures.
Hilary Shinn, 23, of Wall Township, N.J., who likes competitive surfing and
snowboarding, was graduating from Rutgers University last spring and had not
decided whether she would have health insurance.
“I’m a little accident-prone,” said Ms. Shinn, who had a skateboarding accident
on her way to her last class in May. “It didn’t really hit me until my mom
brought it up: ‘Hey, you are not going to be covered when you graduate. Let’s
figure something out.’ ”
The New Jersey law allowed Ms. Shinn, who started graduate school at Monmouth
University this semester, to stay on her father’s plan, for an additional
premium.
In contrast, a number of her friends are now without health insurance. “If they
get sick, they just don’t address it, on the hope it will go away,” she said.
About 30 percent of adults ages 18 to 24, and more than one-quarter of adults 25
to 34, are uninsured, though the average for all age groups is 16 percent,
according to figures released by the Census Bureau in late August.
It is not known how many people have taken advantage of extended coverage,
because policies are administered by private companies and most of the changes
have only recently taken effect.
The rise of uninsured young adults results from two main economic forces,
analysts say. Changes in the workplace mean that fewer jobs now have full
benefits, which disproportionately affects the newest workers. In addition, the
rising cost of premiums, whether shared with an employer or paid individually,
makes insurance less attractive to a relatively healthy population.
“It’s different from a generation ago,” said Katherine Swartz, a professor of
health policy at Harvard University who is the author of “Reinsuring Health,” a
new book on the uninsured population. “We have so many people not used to having
health insurance.”
For years, children have been allowed to stay on their parents’ health insurance
until they turned 19, or until they turned 22 or 23 if they remained full-time
students.
Some of the laws extending the age of coverage allow insurers to charge extra
premiums, which vary depending on the plan. They also have various restrictions,
sometimes requiring that the child be a full-time student, be unmarried, reside
in the state or even live with the parents.
In general, these laws do not apply to insurance plans financed by the employer
— as opposed to plans in which the employer buys coverage from an insurance
company — because self-insured plans, favored by some larger companies, are
shielded from state rules and laws under the 1974 federal Employee Retirement
Income Security Act.
Ms. Shinn’s family pays several hundred dollars a month to add her to her
father’s plan, which is through his employer, Systems Technology, a satellite
communications company. She could have bought insurance on her own for a similar
price, but her father’s plan provides better coverage, said Deborah Shinn, her
mother.
“I know that our children, if they didn’t have family behind them, would never
have an affordable choice,” said Mrs. Shinn, who has four children. “Between car
insurance and just buying books for schools and normal day-to-day expenses, I
really feel that she would never be able to earn enough income to cover the
things that are important for us to have in place for her.”
Before this year, laws extending health coverage were passed in Colorado,
Massachusetts, New Mexico, South Dakota and Texas. Utah, where young Mormon men
commonly complete two years of missionary work, passed the first law, in 1994.
The governors of Delaware and Rhode Island signed such laws last July.
New York State has three bills in legislative committees to raise the age limit
for children to 25, with various restrictions. Connecticut has a similar
proposal in committee.
The availability of health insurance in the workplace has become a problem for
young adults. One reason employers are reluctant to hire full-time employees is
the rising cost of health insurance and other benefits. The percentage of
employers offering health insurance dropped to 60 percent in 2005, down from 66
percent in 2000, according to a survey of employers conducted by the Kaiser
Family Foundation.
Thus, new graduates, from both high school and college, are entering a job
market that is increasingly characterized by consulting, freelance and contract
jobs.
And many employers that still offer insurance are asking employees to shoulder a
higher share of the premiums, another reason young adults decline coverage, said
Laura Tobler, a health policy analyst for the National Conference of State
Legislatures. “It’s a cost-benefit analysis,” she said.
As a result, she said, states are “looking for ways to reach out to these folks
and doing it in the least expensive way possible.”
Brian Merritt, a freelance technology consultant for a Fortune 500 company in
New York City, checked on buying his own health insurance from the Freelancers
Union but decided it was not worth the cost of nearly $200 a month.
“Basically, I’m a healthy 31-year-old male,” he said. “The last three times I
went to the doctor, everything was O.K. So I haven’t felt the need for
insurance.”
He acknowledged the risk. “If I trip on the sidewalk and fall and break my arm,
I guess I am out of luck,” Mr. Merritt said. “Not only am I out medical costs, I
can’t work.”
Other young adults said they had more pressing financial priorities. Tom
Donatelli, a 29-year-old freelance cameraman from Brooklyn, is eager to reduce
his credit-card and other debt. “If I have extra money, then it’s going to go
toward debt that I already have, not debt that I might have,” he said.
Health insurance companies have been supportive of the age extensions that
funnel additional premiums into the system, because they are looking for ways to
keep young adults in the insurance pool, said the assemblyman who sponsored New
Jersey’s bill, Neil M. Cohen. “They can use that new revenue to make adjustments
in the rest of the market,” said Mr. Cohen, a Democrat of Roselle.
Susan Pisano, a spokeswoman for America’s Health Insurance Plans, an industry
group, said that while insurance companies generally did not oppose laws that
extend the age limit by a couple of years, New Jersey’s law, with the age
ceiling of 30, might take it too far.
“The law in New Jersey has the potential to drive up health care costs for
employers, whereas the other ones do not depart as radically from the current
situation in those states,” Ms. Pisano said. “Our members have been comfortable
with what is going on elsewhere.”
In Colorado, however, Leo Tokar, vice president for marketing and sales for
Kaiser Permanente Colorado, said the added expense from that state’s new law
created a “perverse incentive” for companies to reduce insurance benefits.
Employers there can either pass along additional premiums for the adult children
— who can stay on health plans until they reach age 25 — to their parents or
spread the extra cost among all employees.
“Our opinion is it was absolutely the right direction to go, but it was the
wrong way to go about doing it,” Mr. Tokar said.
He said it was inappropriate to make employers that offer insurance serve “as a
conduit to address broader social issues.”
For
Insurance, Adult Children Ride Piggyback, NYT, 17.9.2006,
http://www.nytimes.com/2006/09/17/us/17insure.html
Washing spinach won't help;
health
officials struggle
to find source of E. coli
Updated 9/15/2006 11:46 PM ET
AP
USA Today
WASHINGTON (AP) — Even if you wash the
spinach, you still could be at risk.
Sober warnings for salad lovers came from
federal health officials Friday as they focused on a possible source of a
multistate E. coli outbreak that killed one person and sickened nearly 100 more.
Twenty-nine people have been hospitalized, 14 of them with kidney failure.
The outbreak was traced to Natural Selection Foods, a holding company based in
San Juan Bautista, Calif., known for Earthbound Farm and other brands. The
company has voluntarily recalled products containing spinach.
Food and Drug Administration officials
stressed that the bacteria had not been isolated in products sold by Natural
Selection Foods but that the connection was established by patient accounts of
what they had eaten before becoming ill.
The investigation was continuing.
"It is possible that the recall and the information will extend beyond Natural
Selection Foods and involve other brands and other companies, at other dates,"
said Dr. David Acheson, the chief medical officer with the FDA's Center for Food
Safety and Applied Nutrition.
Natural Selection Foods LLC said in a statement that it was cooperating with
federal and state health officials to identify the source of the contamination
and had stopped shipping all fresh spinach products. They are sold under many
brand names, including Earthbound Farm, Dole, Green Harvest, Natural Selection
Foods, Rave Spinach, Ready Pac and Trader Joe's.
"We are very, very upset about this," Natural Selection Foods spokeswoman
Samantha Cabaluna said Friday night. "What we do is produce food that we want to
be healthy and safe for consumers, so this is a tragedy for us."
Meanwhile, the FDA warned people nationwide not to eat any prepackaged spinach,
whether sold in bags or clamshell boxes. Officials don't know how much
contaminated spinach may be out there, but consumers buy more than 500 million
pounds of it each year.
Washing contaminated spinach won't get rid of the tenacious bug, though thorough
cooking can kill it. Supermarkets across the country pulled spinach from
shelves, and consumers tossed out the leafy green.
"We're waiting for the all-clear. In the meantime, Popeye the Sailor Man and
this family will not be eating bagged spinach," said Dr. William Schaffner,
chairman of preventative medicine at Vanderbilt University. The Tennessee
university's medical center was treating a 17-year-old Kentucky girl for E. coli
infection. That case originally was listed as being from Tennessee, but federal
health officials changed it to Kentucky.
By Friday, the outbreak had grown to include at least 19 states: California,
Connecticut, Idaho, Indiana, Kentucky, Maine, Michigan, Minnesota, New Mexico,
Nevada, New York, Ohio, Oregon, Pennsylvania, Utah, Virginia, Washington,
Wisconsin and Wyoming, according to the Centers for Disease Control and
Prevention. Wisconsin accounted for about half the cases, including the lone
death, Gov. Jim Doyle said.
"We are telling everyone to get rid of fresh bagged spinach right now. Don't
assume anything is over," Doyle said.
In all, the bug is known to have sickened at least 94 people.
FDA officials said they issued the nationwide consumer alert before they could
confirm the source of the tainted spinach.
"Early is good," said Caroline Smith DeWaal, food safety director for the Center
for Science in the Public Interest, adding that the alert may have prevented
hundreds more cases.
An industry spokeswoman said public health concerns justified the blanket
warning: "It needed to happen this way," said Kathy Means, a spokeswoman for the
Produce Marketing Association. "Public health has to trump economics at this
time."
Farmers in California's Monterey County grow more than half the nation's 500
million-pound spinach crop, according to the Agriculture Department.
"We're trying to get to the bottom of this and figure out what happened.
Everybody is terribly concerned," said Dave Kranz, a spokesman for the
California Farm Bureau Federation.
Even before the latest outbreak, a joint state and federal effort has been
underway in the California county to find and eliminate any possible sources of
E. coli contamination.
"We need to strive to do even better so even one life is not lost," said Dr.
Andrew von Eschenbach, FDA's acting commissioner.
The FDA's top food expert stressed the importance of stopping the bacterium at
its source, since rinsing spinach won't eliminate the risk. "If you wash it, it
is not going to get rid of it," said Robert Brackett, director of the agency's
Center for Food Safety and Nutrition.
E. coli lives in the intestines of cattle and other animals and typically is
spread through contamination by fecal material. Brackett said the use of manure
as a fertilizer for produce typically consumed raw, such as spinach, is not in
keeping with good agricultural practices. "It is something we don't want to
see," he told a food policy conference.
Meanwhile, Wal-Mart Stores Inc., Safeway Inc., SuperValu Inc. and other major
grocery chains stopped selling spinach, removing it from shelves and salad bars.
"We pulled everything that we have spinach in," said Dan Brettelle, manager of a
Piggly Wiggly store in Columbia, S.C.
State health officials became aware of the first E. coli poisoning cases on Aug.
25, Acheson said. The FDA was alerted about the outbreak at midweek.
Consumer activist Barb Kowalcyk said fixing the nation's "fractured network" of
food safety agencies could save lives. In 2001, her 2-year-old son, Kevin, died
of E. coli, possibly after eating tainted ground beef.
"How can we improve communication between agencies? That needs to happen," the
Loveland, Ohio, resident said.
Rep. Rosa DeLauro, D-Conn., and other lawmakers seek a hearing on legislation
that would consolidate all federal food safety agencies and establish the Food
Safety Administration, her spokeswoman said. Staff members of the House Energy
and Commerce committee will examine the situation and current government policy,
deputy staff director Larry Neal said.
Not all strains of E. coli cause illness: E. coli O157:H7, the strain involved
in the current outbreak, was first recognized as a cause of illness in 1982.
That strain causes an estimated 73,000 cases of infection, including 61 deaths,
each year in the United States, according to the CDC.
When ingested, the bug can cause diarrhea, often with bloody stools. Most
healthy adults can recover completely within a week, although some people —
including the very young and old — can develop a form of kidney failure that
often leads to death.
Sources of the bacterium include uncooked produce, raw milk, unpasteurized
juice, contaminated water and meat, especially undercooked or raw hamburger.
Anyone who has gotten sick after eating raw packaged spinach should contact a
doctor, officials said. Other bagged vegetables, including prepackaged salads,
apparently are not affected.
"At this point, we are focused on the issue of the spinach. As we learn more, as
we go further, we will alter or change that recommendation," von Eschenbach
said.
Washing spinach won't help; health officials struggle to find source of E. coli,
UT, 15.9.2006,
http://www.usatoday.com/news/health/2006-09-14-ecoli_x.htm
Battle Lines in Treating Depression
September 10, 2006
The New York Times
By BARNABY J. FEDER
TAMARA KNIGHT remembers little of the summer
of 2004 beyond a numbing despair that resisted 16 different antidepressant
drugs. She dreaded a return to electroshock therapy, which she had tried
periodically for years, because it brightened her mood for only a few weeks, at
best, while progressively destroying her memory.
“She was in as dark and low a place as you can ever imagine a person living,”
said Don Knight, her husband. So Ms. Knight drove to a drugstore in her hometown
of Columbus, Ga., bought two large bottles of extra-strength Excedrin and two
boxes of sleeping pills. She said she swallowed as many of the pills as she
could before she passed out.
Katherine V. Coram, another depression sufferer with a history of attempted
suicide, was in relatively better shape that same summer. She had managed to
cling to a job at the National Archives in Washington, where an understanding
boss gave her a light workload. But Ms. Coram felt defeated. Her three years in
a clinical trial to see if an implanted nerve stimulator could control her
illness had ended with hellish results.
“I was hospitalized three times in the year after I got it for anxiety, panic
and other problems — after having gone 15 years without hospitalization,” said
Ms. Coram, who lives alone in Silver Spring, Md. “Once I even hit a stranger in
a restaurant after I got mad. It was totally out of character for me.”
The summer of 2004 was also trying for Cyberonics, a small Houston company that
made Ms. Coram’s stimulator, and Robert P. Cummins, known as Skip, the company’s
combative chief executive. A Food and Drug Administration panel recommended in
June that the agency approve the device — a pocket-watch-sized generator
implanted in the chest that transmits electronic pulses to a major nerve pathway
in the neck — for treating the most intractable forms of depression. But about
two months later, the F.D.A. ignored its own panel’s recommendation and decided
to withhold approval after weighing criticisms from groups opposed to the
controversial treatment.
Since 2004, Ms. Knight and Ms. Coram have continued their struggles with
depression, their fates intersecting with Cyberonics’ own battles with the
F.D.A. and insurers, as well as medical skeptics and public interest groups who
argue that the company is peddling false hopes built on a still unproved
technology.
The struggle over the future of the $15,000 device, which costs another $10,000
or more to implant, is being played out against that most tender of landscapes:
the human psyche and the unpredictable and poorly mapped fault lines that cause
depression and separate people from themselves and the world around them.
IT is a very promising avenue of research, and the long-term data they are
pushing are encouraging,” said Dr. Christopher Gorton, chief medical officer of
APS Healthcare, a consulting firm that was hired by the state of South Carolina
to review Cyberonics’ research results. “But people have a legitimate need to be
cautious. Even the sponsors admit they don’t know exactly how it works. The
psychiatric literature is full of people clutching at straws.”
Some 21 million American adults suffer from depression, according to the
National Institute of Mental Health, a federal research agency. While doctors as
far back as the Renaissance speculated that mood disorders had medical roots, it
was not until the end of the 19th century that such views were widely accepted.
Since then, mental health care has seen innovations in talk therapy,
electroshock, surgical procedures, drugs and, most recently, implanted devices.
Along the way, there have been notorious examples of misplaced medical
enthusiasm, including adoption of lobotomies to treat depression. Currently,
critics complain about frequent misuse of electroshock therapy and the virtually
unregulated mixing of potent antidepression and antipsychotic drugs.
Cyberonics, which finally secured F.D.A. approval to market its implant last
year, has ventured into the most difficult corner of depression treatment. It
says its stimulator can provide relief for many of the four million or so people
who suffer from “treatment resistant depression,” or T.R.D., a form so severe
that patients fail to respond to drugs and traditional shock therapy. No other
product has ever been designed for — and tested exclusively on — such a severely
depressed population.
During the last month, some 1,300 doctors, patients and Cyberonics employees
have written to the Centers for Medicare and Medicaid Services, asking that the
agency grant Cyberonics’ recent petition for Medicare coverage. Public Citizen,
a consumer advocacy group in Washington, filed a competing request on Wednesday,
asking the agency to deny coverage. The group contends that Cyberonics has
relied on misleading advertising and clinical trial write-ups, among other
tactics, to secure federal approvals.
“With substantial constraints on the Medicare budget and so many clear needs
going unmet, it seems absurd to flush away millions of dollars on this unproven
device,” wrote Dr. Peter Lurie, deputy director of health research for Public
Citizen, in the group’s petition. Other problems loom over the company. Federal
prosecutors and regulators are investigating its pay practices, according to
recent securities filings. Earnings disappointments over the last year have also
caused Cyberonics’ stock to drop to $17.30 from a 52-week high of $40.69. Mr.
Cummins declined to comment on the investigations, which are continuing.
But in an e-mail message last week, he scornfully dismissed Public Citizen’s
criticisms as “shrill” and questioned Dr. Lurie’s expertise. “I look forward to
the day when T.R.D. patients and fully qualified psychiatrists have the right to
make treatment decisions regarding the ONLY treatment ever studied, proven safe
and effective and F.D.A. approved for the long-term treatment of T.R.D!” he
wrote.
The Cyberonics implant, like so many modern medical devices born of recent
technological advances, would have been unimaginable only a generation ago.
After being implanted in a patient’s chest, the device is surgically wired to
the neck’s left vagus nerve, a major pathway for nerve signals to the brain from
the heart and torso. Research suggests that repeated electronic stimulation of
the vagus nerve can help treat severe depression by altering chemical and
electrical functions in areas of the brain linked to mood disorders.
Cyberonics, which was founded in 1987, initially developed the stimulator to
help epileptics control seizures. The F.D.A. approved that use in 1997. In 1998,
based on animal tests and reports that many epileptics with the implant
experienced mood improvements, Mr. Cummins gambled Cyberonics’ future on the
idea that the device could also help treat patients with T.R.D. — people like
Ms. Knight and Ms. Coram.
Mr. Cummins forecast, to Wall Street’s delight, that the T.R.D. market might be
10 times bigger than the epilepsy market. But his zeal also reflected his own
family’s experience with depression, an affliction he says drove his mother and
his grandfather to suicide. “My mother was my object lesson in wasted
potential,” he said.
A squarely built, 6-foot-2-inch former college linebacker, Mr. Cummins, 52, says
his mantra is, “Maybe wrong but never in doubt.” His shaved head and nonstop
intensity seem well suited to someone whose idea of relaxation is competing in
Nascar-affiliated car racing events. He now owns a Daytona Prototype race car —
named Cyberspeed in honor of his company.
A native of Grove City, Pa., Mr. Cummins is the youngest of three children born
into a dysfunctional family that he said was ripped apart by the periodic
absences of his father, a steelworker, and his mother’s depression, alcohol and
drug addictions. A decent student and a star athlete, he won a scholarship to
Dartmouth, where he majored in government affairs.
After earning an M.B.A. at the University of Illinois at Urbana-Champaign Mr.
Cummins eventually became a venture capitalist, joining Cyberonics’ board in
1988. Cyberonics was the brainchild of Reese S. Terry Jr., a veteran of the
medical device industry, who set up the company to develop nerve-stimulation
technology invented by Dr. Jacob Zabara at Temple University.
Dr. Zabara was one of many researchers exploring how electrical stimulation of
the nervous system and the brain could affect a range of mental and physical
ailments. The neurostimulation market as a whole now generates $1 billion
annually in revenue, according to Wall Street analysts, and includes devices
like spinal-cord stimulators that mask pain and deep brain implants that control
some symptoms of Parkinson’s disease.
Wall Street and leading device companies see neurostimulation growing into a $10
billion business in the next decade, thanks to rapid advances in microchips and
sensors. Executives at Cyberonics say that focusing on the vagus nerve makes its
implants as effective but less risky and invasive than those that stimulate the
brain and spinal cord.
Mr. Cummins became Cyberonics’ chief executive in 1995, when the company was
struggling to stay afloat financially while seeking regulatory approval of its
epilepsy therapy. He took over about the same time that Tamara Knight began a
seemingly irreversible slide into severe depression.
Ms. Knight began suffering from depression as a teenager, a struggle that led to
an early suicide attempt. Several years ago, drugs that had helped her recover
stopped working. She began to neglect housework and her three children. In the
fall of 1998, after several incidents in which she became outraged with
co-workers, she lost her job. That winter, she began electroshock therapy.
What followed, she said, were more than 30 such treatments and more
hospitalizations than she can recall. The number of drugs she took kept
increasing, along with her weight. At one point, Ms. Knight, who is 5-foot-6 and
had struggled with anorexia as a teenager, weighed more than 200 pounds. By
2004, she was depressed and was suffering from a common side effect of the
electroshock therapy required to control her suicidal impulses: a steady erosion
of her memory.
“Moneywise, insurance and Medicare paid for them,” Ms. Knight said of her
treatments. “But it cost 30 years of my life in memory loss.”
As often happens in cases of severe depression, the family says it also lost the
support of outsiders, including its local church. “If Tamara had had cancer, our
church would have had people visiting all the time,” Mr. Knight said. “One older
man visited us as long as he was able. That was it. It was a real blow to our
faith.”
When Ms. Knight’s psychiatrist recommended a Cyberonics implant shortly after
the F.D.A. approved the device in 2005, she became enthusiastic. Mr. Knight, who
said he thought that V.N.S. therapy — Cyberonics’ trademarked name for “vagus
nerve stimulation” — sounded far-fetched when he heard about it a few years
earlier, now agreed that it was a chance they had to take.
HE was worried, though, that public information about V.N.S. came largely from
Cyberonics and highlighted favorable aspects of clinical data. Negative
experiences of clinical-study patients like Katherine Coram were not readily
available, outside of commentaries that epilepsy patients posted on a Cyberonics
Web site.
Ms. Coram recalls feeling better in the first six months after her Cyberonics
stimulator first turned on in 2001.
But when researchers ramped up her implant’s power to enhance its long-term
performance, Ms. Coram began suffering nausea, sleeplessness and panic attacks
that sent her to the hospital. She lost track of her finances. Like many victims
of severe depression, she also began to experience distressingly peculiar
setbacks, including an inability to load her dishwasher. Ms. Coram said doctors
raised and lowered the implant’s power level several times before she asked them
to turn it off temporarily.
“I was trusting the people in the study to figure out how to use the device but
they didn’t have a clue,” Ms. Coram said.
The experiences of individuals like Ms. Coram and inconclusive data were not
enough, though, for the F.D.A. to shut its doors to Cyberonics. In June 2004, an
advisory panel recommended that the agency allow Cyberonics to market its
stimulator.
That same night, Cyberonics’ board granted Mr. Cummins a bonus of 150,000 stock
options. The next day, as the stock market reacted to the F.D.A. panel’s
recommendation, Cyberonics shares leapt 77 percent, laying the groundwork for
what, two years later, would become inquiries by the Securities and Exchange
Commission and the Justice Department into whether Mr. Cummins’s options grant
was legal.
But in 2004, Mr. Cummins had more immediate problems. By early August, critics
inside and outside the F.D.A. had talked the agency into the unusual step of
disagreeing with its advisory panel. True, there were success stories from
patients who achieved almost complete recoveries (nearly 20 percent, according
to Cyberonics). Data also suggested that patients who benefited — over 50
percent to one degree or another — were more likely to avoid relapses, expensive
hospitalizations and suicide than a similar group of patients who received
conventional therapy.
Still, in the most carefully controlled trial, a group that had the device
implanted but not turned on fared nearly as well as the group being stimulated.
Critics also pointed out that long-term results indicated that 30 percent of the
patients reported worsening depression similar to Ms. Coram’s, creating
unanswered questions about potential harm.
Mr. Cummins said in a press release that Cyberonics was “shocked” by the
agency’s rejection. He denigrated the F.D.A. request for more randomized
testing, arguing that other antidepression therapies had been subjected to far
less challenging scrutiny. And he suggested to analysts that the agency’s
decision would leave many patients feeling that they had no option other than
suicide. In September, the F.D.A. accepted an amended application from
Cyberonics that contained more long-term performance data and comparisons to
other therapies.
Still, it came as something of a surprise on Wall Street and in the medical
device industry when the F.D.A. decided early in February 2005 to approve V.N.S.
as a depression treatment if Cyberonics met certain conditions. Most of the new
requirements dealt with follow-up studies aimed at figuring out how the device
could be used most effectively. Mr. Cummins forecast that all of the conditions
could be met by the end of May. Shares of Cyberonics soared, and the company
went on a hiring spree.
Mr. Cummins cast the decision as a victory for patients but also heralded the
financial upside, projecting that Cyberonics’ sales could reach $1 billion by
2010. In the weeks after the F.D.A.’s announcement, he responded to Cyberonics’
stock run-up by exercising options on 350,000 shares, which netted him about
$10.75 million. (The sales left him holding just over 36,000 Cyberonics shares,
a stake he has since raised to 173,750 shares, according to federal filings.)
The long-awaited marketing approval from the F.D.A. came in July, but new
problems emerged. A prominent board member resigned, citing disagreements with
other directors over compensation policies and lack of a succession plan for Mr.
Cummins.
The Senate Finance Committee, which began investigating the F.D.A.’s handling of
the implant that spring, issued a staffreport in February of this year
criticizing Dr. Daniel G. Schultz, a senior F.D.A. official, for overruling
agency experts opposed to the device. An F.D.A. spokeswoman said that the agency
acted carefully and that for “the small number of patients with severe
depression who have failed all other treatments, data shows this may be the
difference between being able to live a more productive life or suffer from
debilitating illness.”
The Senate report also put Medicare officials on notice that decisions to cover
V.N.S. would invite further scrutiny.
Continued uncertainty surrounding V.N.S. and Mr. Cummins’s us-against-the-world
attitude have taken their toll on Cyberonics’ stock price and on Wall Street’s
faith in the company. “There are institutional investors who won’t touch the
company as long as Skip is at the helm,” said Jan Wald, an analyst at A. G.
Edwards & Sons.
To hear Mr. Cummins tell it, what really matters is that a growing number of
depression patients now credit the device with saving their lives. They include
Ms. Knight, who got her Cyberonics implant last October, and quickly proved to
be the kind of patient the company treasures.
“My family could see subtle differences right away,” Ms. Knight recalled in the
letter of support for V.N.S. she sent to Medicare regulators last month. “I was
out of bed more often and started taking an interest in how the house looked.”
While Ms. Knight, now 47, talks about “having my life back,” the legacy of
severe depression remains with her. After spending so much time for so many
years in bed, her body cannot keep up with all the things she now wants to do
around the house, often leaving her in pain. She continues to lose her way
around her hometown and mourns the loss of so much of her long-term memory. But
she has been able to cut back to using just three medications, a change she
credits with helping her shed 40 pounds.
MS. CORAM is also on the mend, but not because of V.N.S. or Cyberonics. After
enduring numerous futile adjustments to her implant and having it temporarily
turned off, she asked her doctors to try one last time in March 2004. Three
months later, she stopped using the implant for good and then had it removed
from her chest. Her doctors left the metal leads wrapped around her vagus nerve,
saying it was too risky to try to remove them.
She said she is slowly regaining the ability to do household tasks — like paying
bills — that had been impossible during her V.N.S. treatment. “I feel like I’m
just beginning to get my life back,” she said recently.
Mr. Cummins remains as driven as ever. Cyberonics says 1,810 patients have
received the implants since the product was approved, out of 13,313 identified
candidates. Thousands have been caught up in lengthy and often unsuccessful
appeals for insurance coverage, since only a few small insurers routinely pay
for the device.
Seeking the traction it desperately needs with insurers, Cyberonics decided in
May to narrow the company’s focus to the subset of T.R.D. patients whose
problems are the most costly for health care providers. Cyberonics contends that
V.N.S. treatment for that group would save hospitals $4,800 to $8,200 a patient
annually, based on an estimate of the implant’s cost and follow-up visits over
eight years. But Cyberonics’ calculation is based on optimistic presumptions,
including aggressive estimates about the pace and nature of patients’ recovery.
Because the presumptions are also based primarily on results from earlier
trials, critics like Public Citizen say the new focus has all the flaws of the
old one.
However the controversy is resolved, depression victims who finally get an
implant will find themselves entering a medical lottery. Many, like Joe
Marhefka, 49, of Colchester, Conn., are too depressed to be optimistic about the
procedure.
Mr. Marhefka, who ended up in the hospital last fall after attempting suicide,
said that he masked his depression most of his life by drinking. When he gave up
alcohol eight years ago, the depression seemed to intensify. He said
electroshock therapy two years ago destroyed most of his memories of his
adolescence, and younger adult years. He lost his job as a high school math
teacher shortly before his suicide attempt.
“When I had the implant last month, I was hoping I wouldn’t wake up,” Mr.
Marhefka said in July, before entering the New London, Conn., office of Dr.
Kathleen R. Degen to have his stimulator activated. “I don’t have any hopes this
is going to work. I’m doing this because my wife deserves a husband and my two
daughters deserve a Dad. They have hope. I’m just going along with the doctors.”
Dr. Degen used a hand-held computer to program a wand that Mr. Marhefka held
over the spot on his chest where his implant was located. A 30-second-long pulse
surged into his vagus nerve, reducing Mr. Marhefka’s voice to a barely audible
rasp. When the pulse stopped, his voice returned to normal and he conversed
briefly with Dr. Degen. He asked about reducing his medications; Dr. Degen said
the request was common, but that it was too early to consider it.
Five minutes later, just as Mr. Marhefka asked whether his implant was still on,
it delivered its second pulse — again taking away his voice and leaving him with
what he said was a mild strangling feeling. Dr. Degen let the pulse pass and
explained how she would gauge his future progress.
But Mr. Marhefka plans to measure his progress with his own yardstick.
“If I start smiling again, maybe even laughing sometimes, that’s how I’ll know,”
he had said just before entering Dr. Degen’s office. So far, Mr. Marhefka said
in a phone call last week, he is still waiting.
Battle Lines in Treating Depression, NYT, 10.9.2006,
http://www.nytimes.com/2006/09/10/business/yourmoney/10cyber.html
Mental Activity
Seen in a Brain Gravely
Injured
September 8, 2006
The New York Times
By BENEDICT CAREY
A severely brain-damaged woman in an
unresponsive, vegetative state showed clear signs on brain imaging tests that
she was aware of herself and her surroundings, researchers are reporting today,
in a finding that could have far-reaching consequences for how unconscious
patients are cared for and how their conditions are diagnosed.
In response to commands, the patient’s brain flared with activity, lighting the
same language and movement-planning regions that are active when healthy people
hear the commands. Previous studies had found similar activity in partly
conscious patients, who occasionally respond to commands, but never before in
someone who was totally unresponsive.
Neurologists cautioned that the new report characterized only a single, perhaps
unique case and that it did not mean that unresponsive brain-damaged people were
more likely to recover or that treatment was possible. The woman in the study
could not communicate with the researchers, and there was no way to know whether
her subjective experience was anything like what healthy people call
consciousness. The woman was injured in a traffic accident in England last year.
Yet the study so drastically contradicted the woman’s diagnosed condition that
it exposed the limitations of standard methods of bedside diagnosis. And its
findings are bound to raise hopes for tens of thousands of families caring for
unresponsive, brain-damaged patients around the world — whether those hopes are
justified or not, experts said.
“One always hesitates to make a lot out of a single case, but what this study
shows me is that there may be more going on in terms of patients’ self-awareness
than we can learn at the bedside,” said Dr. James Bernat, a professor of
neurology at the Dartmouth Medical School, who was not involved in the study.
“Even though we might assume some patients are not aware, I think we should
always talk to them, always explain what’s going on, always make them
comfortable, because maybe they are there, inside, aware of everything.”
Dr. Bernat added that brain imaging promised to improve the diagnosis of
unconscious states in certain patients, but that the prospect of imaging could
also raise false hopes in cases like that of Terri Schiavo, the Florida woman
who was removed from life support and died last year after a bitter national
debate over patients’ rights.
Ms. Schiavo suffered far more profound brain damage than the woman in the study
and was unresponsive for some 15 years, according to neurologists who examined
her.
The journal that published the new paper, Science, promoted the finding in a
news release, but added a “special note” citing the Schiavo case and warning
that the finding “should not be used to generalize about all other patients in a
vegetative state, particularly since each case may involve a different type of
injury.”
The brain researchers, led by Adrian Owen at the Medical Research Council
Cognition and Brain Sciences Unit in Cambridge, England, performed scans on the
patient’s brain five months after her accident. The imaging technique, called
functional M.R.I., reveals changes in activity in specific brain regions. When
the researchers spoke sentences to the patient, language areas in her brain
spiked in the same way healthy volunteers’ did.
When presented with sentences containing ambiguous words, like “The creak came
from a beam in the ceiling,” additional language processing areas also became
active, as in normal brains. And when the researchers asked the woman to imagine
playing tennis or walking through her house, they saw peaks of activity in the
premotor cortex and other areas of her brain that mimicked those of healthy
volunteers.
“If you put her scans together with the other 12 volunteers tested, you cannot
tell which is the patient’s,” Dr. Owen said in an interview. Doctors from the
University of Cambridge and the University of Liège in Belgium collaborated on
the research.
Dr. Nicholas Schiff, a neuroscientist at Weill Cornell Medical College in New
York, said that the study provided “knock-down, drag-out” evidence for mental
awareness, but that it was not clear “whether we’ll see this in one out of 100
vegetative patients, or one out of 1,000, or ever again.”
In a more recent exam, more than 11 months after her injury, the patient
exhibited a sign of responsiveness: she tracked with her eyes a small mirror, as
it was moved slowly to her right, and could fixate on objects for more than five
seconds, said Dr. Steven Laureys, a neurologist at the University of Liège and
an author of the study. This means by definition that the young woman has
transitioned from an unresponsive, vegetative state to a sometimes responsive
condition known as a minimally conscious state, Dr. Laureys said in an
interview. An estimated 100,000 Americans exist in this state of partial
consciousness, and some of them eventually regain full awareness.
The chances that an unresponsive, brain-damaged patient will eventually emerge
depend on the type of injury suffered, and on the length of time he or she has
been unresponsive. Traumatic injuries to the head, often from car accidents,
tend to sever brain cell connections and leave many neurons intact. About 50
percent of people with such injuries recover some awareness in the first year
after the injury, studies find; very few do so afterward. By contrast, brains
starved of oxygen — like that of Ms. Schiavo, whose heart stopped temporarily —
often suffer a massive loss of neurons, leaving virtually nothing unharmed. Only
15 percent of people who suffer brain damage from oxygen deprivation recover
some awareness within the first three months. Very few do after that, and a 1994
review of more than 700 vegetative patients found that none had done so after
two years.
The imaging techniques used in the new study could help identify which patients
are most likely to emerge — once the tests are studied in larger numbers of
unconscious people, said Dr. Joseph Fins, chief of the medical ethics division
of New York Presbyterian Hospital-Weill Cornell Medical Center.
Without this context, Dr. Fins said, the imaging tests could create some
confusion, because like any medical tests they may occasionally go wrong,
misidentifying patients as exhibiting consciousness or lacking it. “For now I
think what this study does is to create another shade of gray in the
understanding of gray matter,” he said.
Mental Activity Seen in a Brain Gravely Injured, NYT, 8.9.2006,
http://www.nytimes.com/2006/09/08/science/08brain.html
Gene Called Link
Between Life Span and
Cancers
September 7, 2006
The New York Times
By NICHOLAS WADE
Biologists have uncovered a deep link between
life span and cancer in the form of a gene that switches off stem cells as a
person ages.
The critical gene, well known for its role in suppressing tumors, seems to
mediate a profound balance between life and death. It weighs the generation of
new replacement cells, required for continued life, against the risk of death
from cancer, which is the inevitable outcome of letting cells divide.
To offset the increasing risk of cancer as a person ages, the gene gradually
reduces the ability of stem cells to proliferate.
The new finding, reported by three groups of researchers online yesterday in
Nature, was made in a special breed of mice that lack the pivotal gene, but is
thought likely to apply to people, as well.
The finding suggests that many degenerative diseases of aging are caused by an
active shutting down of the stem cells that renew the body’s various tissues and
are not just a passive disintegration of tissues under daily wear and tear. “I
don’t think aging is a random process — it’s a program, an anticancer program,”
said Dr. Norman E. Sharpless of the University of North Carolina, senior author
of one of the three reports.
The other senior authors are Drs. Sean J. Morrison of the University of Michigan
and David T. Scadden of the Harvard Medical School.
The full implications are far from clear, but the finding that the cells are
switched off with age does not seem too encouraging for researchers who hope to
use a patient’s own adult stem cells to treat disease. That result may undercut
opponents of research on human embryonic stem cells who argue that adult stem
cells are enough to build new tissue.
Dr. Sharpless said his finding showed the need to pursue both types of research.
The gene in the finding has the unmemorable name of p16-Ink4a. It plays a
central role in the body’s defenses against cancer, and it produces two quite
different proteins that interact with the two principal systems for deciding
whether a cell will be allowed to divide.
One of the proteins had also been noted to increase substantially with age. The
cells of a 70-year-old produce 10 times as much of the Ink4 protein as those of
a 20-year-old, Dr. Sharpless said.
To help understand that Dr. Sharpless genetically engineered a mouse strain with
the gene knocked out. He set out to see whether losing the gene would affect the
blood-making stem cells. Learning that Dr. Morrison was interested in that with
brain cells and Dr. Scadden with the insulin-making cells of the pancreas, he
shared his mice with them.
The teams agreed to publish their findings together, a departure from usual
researchers’ competitiveness.
All three teams report essentially the same results, that in each type of tissue
the cells have extra ability to proliferate when the Ink4 protein can no longer
be made.
At the same time, the Ink-less mice are highly prone to cancer, which they start
to develop as early as 1 year of age.
The researchers assume, but have not yet proved, that the increasing amounts of
Ink4 as a person ages will thrust the stem cells into senescence, meaning that
they can never divide again. The evolutionary purpose is evidently to avert the
risk that a damaged stem cell might evade controls and proliferate into a tumor.
One implication is that therapists who hope to increase longevity have to tackle
a system that may be hard to cheat. An intervention that reduces Ink4 production
to prevent the age-related decline of stem cells will also increase the risk of
cancer.
“There is no free lunch,’’ Dr. Sharpless said. “We are all doomed.”
He quickly modified that by noting that a calorically restricted diet is one
intervention that is known to increase life span and reduce cancer, at least in
laboratory mice. The reason, he said, is probably because such diets reduce cell
division, the prime source of cancer risk.
For cell therapists, the dual activity of Ink4 may be “a hard box to get out
of,” he said, unless they use cells that are somehow much younger than the
patient.
Dr. Scadden, however, said he hoped that there would turn out to be some
sloppiness in the Ink4 gene’s balancing trick, allowing it to be switched off
temporarily with yet-to-be invented drugs in a way that would promote stem cell
proliferation without greatly increasing the risk of cancer. “There is clearly a
dark side to the finding, but whether or not we can exploit it, that’s the
challenge,” he said.
Some proposals for stem cell therapy with adult stem cells envisage taking a
patient’s stem cells, making them divide in the laboratory and putting them back
in the patient to build new tissue.
“The notion that adult stem cells are infinite in proliferative capacity is
seriously undermined by this work,” Dr. Sharpless said.
Dr. Morrison said it had long been known that older patients did not do as well
in bone marrow transplants as younger ones, and the new finding might explain
why.
“I don’t think any of these findings dims the promise of stem cell research at
all,” he said, because the greater robustness of younger people’s cells was
already well known.
The researchers said they did not yet know what stimulus makes cells increase
their production of the Ink4 protein as a person grows older. Their suspicion is
that the usual factors implicated in aging like mutation and oxidative damage to
tissues would turn out to have a role in making cells produce more Ink4.
Dr. Ronald A. DePinho, an expert on cellular aging and a co-author of Dr.
Scadden’s report, said the new finding, in showing how the renewal capacity of
stem cells was governed, might enable drugs to be made that would improve cell
transplants.
Dr. Scott Lowe, a cancer gene expert at the Cold Spring Harbor Laboratory on
Long Island who was not involved in the three papers, said the results were
interesting because they linked to aging a gene of central importance in cancer.
Press releases by the University of North Carolina School of Medicine and the
University of Michigan attributed the advance to all three teams equally. But
the press release issued by the Harvard Stem Cell Institute, where Dr. Scadden
has an appointment, described him as the leader of the multi-institutional team,
with the other two teams confirming his work. Dr. Scadden made no such claim in
an interview, and acknowledged Dr. Sharpless’s generosity in lending his mice.
Gene
Called Link Between Life Span and Cancers, NYT, 7.9.2006,
http://www.nytimes.com/2006/09/07/science/07stem.html
NYT
2.9.2006
Seeking Healthy Children, Couples Cull
Embryos NYT
3.9.2006
http://www.nytimes.com/2006/09/03/health/03gene.web.html
The DNA Age
Seeking Healthy
Children,
Couples Cull Embryos
September 3, 2006
The New York Times
By AMY HARMON
As Chad Kingsbury watches his daughter playing in the
sandbox behind their suburban Chicago house, the thought that has flashed
through his mind a million times in her two years of life comes again: Chloe
will never be sick.
Not, at least, with the inherited form of colon cancer that has devastated his
family, killing his mother, her father and her two brothers, and that he too may
face because of a genetic mutation that makes him unusually susceptible.
By subjecting Chloe to a genetic test when she was an eight-cell embryo in a
petri dish, Mr. Kingsbury and his wife, Colby, were able to determine that she
did not harbor the defective gene. That was the reason they selected her, from
among the other embryos they had conceived through elective in vitro
fertilization, to implant in her mother’s uterus.
Prospective parents have been using the procedure, known as preimplantation
genetic diagnosis, or P.G.D., for more than a decade to screen for genes certain
to cause childhood diseases that are severe and largely untreatable.
Now a growing number of couples like the Kingsburys are crossing a new threshold
for parental intervention in the genetic makeup of their offspring: They are
using P.G.D. to detect a predisposition to cancers that may or may not develop
later in life, and are often treatable if they do.
For most parents who have used preimplantation diagnosis, the burden of playing
God has been trumped by the near certainty that diseases like cystic fibrosis
and sickle cell anemia will afflict the children who carry the genetic mutation
that causes them. The procedure has also been used to avoid passing on
Huntington’s disease, a severe neurological disease that typically doesn’t
surface until middle age but spares no one who carries the mutation that causes
it.
Couples like the Kingsburys, by contrast, face an even more complex calibration.
They must weigh whether their desire to prevent suffering that is not certain to
occur justifies the conscious selection of an embryo and the implicit rejection
of those that carry the defective gene.
As doctors and genetic counselors at leading cancer centers like Memorial
Sloan-Kettering in New York start to suggest the possibility of P.G.D., more
young patients are finding that their answer lies in trading natural conception
for the degree of scientific control offered by the procedure. And if the
growing interest in screening for cancer risk signals an expanded tolerance for
genetic selection, geneticists and fertility experts say it may well be
accompanied by the greater use of preimplantation diagnosis to select for
characteristics that range from less serious diseases to purely matters of
preference.
Already, it is possible to test embryos for an inherited form of deafness or a
mild skin condition, or for a predisposition to arthritis or obesity. Some
clinics test for gender. As scientists learn more about the genetic basis for
inherited traits, and as people learn more about their genetic makeup, the
embryo screening menu and its array of ethical dilemmas are only expected to
grow.
“From a technology perspective we can test anything,” said Mark Hughes, director
of the Genesis Genetics Institute in Detroit, who is performing P.G.D. this
month for two couples who want to avoid passing on a susceptibility to breast
cancer. “The issue becomes what is considered serious enough to warrant such
testing and who decides that.”
The process is also difficult and expensive. P.G.D., which requires in vitro
fertilization, can cost tens of thousands of dollars. While insurance companies
often pay for the more traditional uses of the procedure, they have not done so
for cancer-risk genes, fertility experts say. The barrier to affordability, some
critics fear, could make preimplantation diagnosis for cancer risk the first
significant step toward a genetic class divide in which the wealthy will become
more genetically pure than the poor.
Knowing that Mr. Kingsbury had tested positive for the colon cancer mutation,
the Kingsburys started with the basic laws of genetics: because children
randomly inherit half of each parent’s genes, he had a 50 percent chance of
passing it on. Since the mutation raises the risk of developing the cancer by
about twentyfold, that means any child of theirs conceived the traditional way
would have about a one in three chance of getting it, usually around age 45.
Those who did develop the cancer would also have a nearly 90 percent chance of
surviving it, but only if it was caught early.
The jumble of odds meant little to the Kingsburys as they tried to think about
starting a family while the cancer claimed Mr. Kingsbury’s second uncle. Then,
from a cousin of Mr. Kingsbury’s who had also fought colon cancer, they heard
about P.G.D., the technology that offered them a way to reload the genetic dice.
To do it, they had to overcome their own misgivings about meddling with nature.
They had to listen to the religious concerns of Mr. Kingsbury’s family and to
the insistence of Ms. Kingsbury’s that the expense and physical demands of in
vitro fertilization were not worth it, given that the couple could probably get
pregnant without it. They had to stop asking themselves the unanswerable
question of whether a cure would be found by the time their child grew up.
It took them two months to make the decision. But every time Mr. Kingsbury looks
at Chloe, with her blue saucer eyes and her tantrums that turn abruptly to
laughter — and back — he knows it was worth it.
“I couldn’t imagine them telling me my daughter has cancer,” he said, “when I
could have stopped it.”
Shifting Medical Advice
Cancer-prone families are only just beginning to hear about P.G.D. in part
because the procedure falls through the cracks in medicine. Oncologists tend not
to think about family planning, and obstetricians tend not to think about cancer
genetics. Doctors may also shy from raising the prospect of what some critics
call “unnatural selection.”
For many couples, going to such lengths to ensure that a child will be born free
of a predisposition to a certain kind of cancer is anathema. Breast and colon
cancer, the two most common cancers for which genetic susceptibility tests are
available, can be detected early and are often treatable, and even those who die
of them often lead long and productive lives.
Many people without the risk-raising genes still get one of the cancers, and
those who do carry the genetic mutations are just as likely as anyone else to
develop other forms of the disease.
Prospective parents who want to avail themselves of P.G.D. must first undergo
the same in vitro fertilization process often used to assist infertile couples,
in which eggs are extracted from the mother and fertilized with the father’s
sperm in a petri dish. When the resulting embryos are three days old, doctors
remove a single cell from each and analyze its DNA. Only embryos without the
defective gene are then considered candidates to implant in the mother’s uterus.
The out-of-pocket costs often exceed $25,000, depending on how many in vitro
cycles are required. Because embryos are selected for their genetic status,
rather than solely by which look the healthiest, the chance that they will fail
to develop after implantation is higher. And despite the birth of thousands of
apparently healthy babies after P.G.D., there is still concern that the
long-term effects of removing a cell from an eight-cell embryo have not been
studied enough.
That has not stemmed the rising demand to screen embryos for cancer-risk genes.
No one tracks the number of such procedures in the United States, but an article
in next month’s issue of The Journal of Clinical Oncology reports that clinics
around the country have been quietly performing P.G.D. for hereditary cancers of
the breast and the colon. The article, written by Dr. Kenneth Offit, chief of
clinical genetics at Memorial Sloan-Kettering Cancer Center, suggests there
would be even more interest in P.G.D. if oncologists were to begin informing
prospective patients of the option.
About one in every 200 Americans carry a genetic mutation that makes them more
susceptible to breast or colon cancer. The half-million or so who carry
mutations in several genes associated with colon cancer have up to a 70 percent
chance of developing the disease, compared with 6 percent for those with normal
copies of the genes. One in eight American women will develop breast cancer in
their lifetimes, but for those who carry mutant forms of the BRCA1 or BRCA2
genes, the risk jumps to one in two.
Until the last year or so, Dr. Offit said in an interview, he questioned the
utility of P.G.D. for his patients, focusing instead on the increasingly
effective prevention and treatment options for anyone born with an elevated
cancer risk. Genetics, he said, is not necessarily destiny.
But learning of its existence, Dr. Offit noticed, could make many young patients
less fatalistic about their genes and more optimistic about starting a family.
The sense of relief it offered was especially strong among those who had seen a
parent or a sibling die of cancer.
“Having seen so many children from cancer-prone families, I’m more sensitive to
the sentiment that they would rather avoid the syndrome altogether,” Dr. Offit
said. “Our genetic counselors now try to bring up the potential of this
technology in circumstances where we think it may be empowering to young
couples.”
Too Late for Some Carriers
If other cancer centers follow Sloan-Kettering’s lead, the shift will still come
too late for some cancer gene carriers. One woman, a professional in New York,
said she blamed bad medical advice for her having possibly passed to her
4-year-old daughter a chance of developing breast cancer this is five times as
great as that of women in the general population.
Unaware of P.G.D., she followed the counsel given to most women with a family
history of breast cancer and had her children before getting tested for the
gene. The logic is that women ought not worry about their genetic status until
they can consider the most effective prophylactic measures, removing their
breasts and ovaries.
But in postings to an online breast cancer support group, facingourrisk.org, the
woman said she suspected that the prevailing unease over genetic selection and
the question of when life begins also kept doctors from suggesting P.G.D.
“Those values should not be dictating recommendations by doctors,” said the
woman, 40, who declined to be identified by name because her words might be
hurtful to her family. “That’s what I resent. I feel like the choice was taken
away from me.”
In Europe, divergent values are quite explicitly shaping different P.G.D.
policies. This spring, England approved the use of preimplantation diagnosis for
the breast and colon cancer risk genes. In Italy, the procedure has been
effectively banned for any condition.
In the United States, where the technology is not regulated, decisions about
when it is appropriate are left largely to fertility specialists and their
patients. Reflecting the growing demand for the procedure, the company that owns
the tests for the breast cancer genes recently licensed the right to use them to
three fertility centers.
“We decided we don’t want to do it here,” said William Hockett, a spokesman for
the company, Myriad Genetics of Salt Lake City, citing both moral preferences
and a disinclination to invest in the technique for business reasons. “But we
don’t want to impose our values on society, so we felt we should allow others to
do it if they wished.”
The interest in embryo testing is being driven largely by a greater knowledge of
genetics among cancer patients and their family members. In the last five years,
nearly 10 times as many Americans have been tested for the breast-cancer risk
genes as in the previous five, according to Myriad, surpassing a total of
100,000 since the test was made available in 1996.
Familiarity with their own genetic profile makes some people more comfortable
with intervening to alter their children’s. For them, genetic traits can seem
less like destiny and more like any other part of their lives that can be
improved by technology.
Many of those exploring P.G.D. are the first generation of women to have reached
reproductive age after their mothers developed cancer and tested positive for
one of the breast cancer mutations. They see it as saving not just their
children but generations of descendants from the same fate.
“I was very relieved to know that I would not have to pass this gene on to my
children,” said Michaela Walsh, 20, a junior at Susquehanna University in
Selinsgrove, Pa., who found out she carries a BRCA mutation. She has already
decided she wants to use P.G.D. when she has children. “My mother told me that
the only worse thing than having cancer twice was having to give the gene to
me.”
But the same knowledge makes others who carry the mutations take particular
offense at the selection procedure, which they say implies that they themselves,
and many members of their family, should never have existed. It raises the
specter of eugenics, they say, in the most personal terms.
“It’s like children are admitted to a family only if they pass the test,” said
Denise Toeckes, 32, a teacher who tested positive for a BRCA mutation. “It’s
like, ‘If you have a gene, we don’t want you; if you have the potential to
develop cancer, you can’t be in our family.’ ”
Other critics oppose preimplantation diagnosis on the grounds that it could be
used to select against homosexuals, women or people with disabilities. It
reduces people to their genes, they say, and paves the way for the pursuit of
children designed to suit parental ideals and for discrimination against those
born with perceived imperfections.
Proponents of the technology say that confusing the concept of “designer babies”
with people trying to avoid deadly illnesses is hurtful and misleading. No one,
they say, would endure the substantial physical and emotional difficulty posed
by the process to make a baby with blue eyes and a wicked curveball. Still, the
hostility couples have encountered from friends, family, colleagues and even
medical professionals caused several of those interviewed for this article to
request that their names be withheld.
One prospective father, a medical resident at Johns Hopkins Hospital in
Maryland, said the Shady Grove Fertility Center, the local fertility clinic,
required that he and his wife, also a resident at Hopkins, write a letter
justifying their request for P.G.D. to the clinic’s ethics committee.
The doctor’s own father continually warned that in trying to prevent cancer,
removing a cell from the embryo would create a mentally retarded child — no
matter how many times his son cited studies to the contrary. Reactions from
colleagues made the couple worry that if they allowed their names to be used, it
might hurt their chances when applying for jobs at medical or research
institutions.
“It became such a negative topic of conversation that my wife and I decided,
‘We’re not talking about this to anyone,’ ” said the doctor, 30, whose mother
has had her breasts, uterus and ovaries removed to combat her cancer, in
addition to undergoing dozens of chemotherapy sessions.
The couple held firm in their belief that there was no virtue in letting nature
take its course when its outcome was so potentially damaging. “We hope to look
back on this as really the first decision we made as parents,” they wrote in a
letter to the ethics committee that persuaded the clinic to let them move ahead.
Multiplying Decisions
Even for those who choose it, the burden of selection weighs heavily. Kim
Surkan, who carries the BRCA1 breast cancer mutation, said her partner had
initially described preimplantation diagnosis as a “pact with the devil.” As Ms.
Surkan prepares for her eggs to be extracted next month, she has nightmares that
the child she selects will drown in a swimming pool, as opposed to a child
chosen by fate, who might carry the cancer-risk gene but would have been a good
swimmer.
“At least I know I’ve done whatever I can do with the information I have,” said
Ms. Surkan, an adjunct lecturer in women’s studies at the Massachusetts
Institute of Technology. “I can’t control everything.”
Like many people who want to take advantage of P.G.D. but cannot easily afford
it, Ms. Surkan had to first convince her insurance company that she was
infertile so that it would pay for the in vitro fertilization process. Because
she is in a same-sex couple, that meant she had to undergo several cycles of
insemination, hoping that she would in fact not get pregnant, so that she could
proceed with preimplantation diagnosis.
Now more decisions are coming. What if, she wonders, she is unlucky, and all her
embryos have disease genes? Should she implant a male one, since men rarely
develop breast cancer, even if she is opposed to selection based on gender?
If she does get pregnant with an embryo found to be free of the gene, should she
test the fetus at 16 weeks, since there is up to a 3 percent chance that P.G.D.
will fail to detect an unwanted mutation? If she has two embryos implanted, and
one has the defective gene, should she terminate it?
For many couples, preimplantation diagnosis is an appealing option precisely
because it does not require terminating a pregnancy, a step that is common after
an amniocentesis reveals that a fetus has a severe genetic disease but is
essentially unheard of for predisposition to common cancers.
Danielle Jamond, who carries a gene for a severe form of inherited colon cancer,
said she and her husband were considering that option a year ago. But she was
saved from making the choice when she heard of a doctor who had developed a
P.G.D. procedure for her form of the disease.
“At that point, the choice was obvious,” said Ms. Jamond, a human resources
manager in a suburb of Paris, who has had her large intestines removed to avoid
getting the cancer, a prophylactic measure for people with her genetic mutation.
Four embryos were created from her 12 eggs, and three did not have the genetic
mutation. One died, the other two were implanted, and one survived. She is now
six months pregnant with the surviving one, a girl.
But some people who believe life begins with conception think P.G.D. is as
unethical as abortion and perhaps more pernicious because it is psychologically
less burdensome. Unused embryos may be frozen indefinitely, skirting one moral
issue, but at a cost of several hundred dollars a year. Reproductive Genetics
Institute, a leading P.G.D. lab in Chicago that performed the preimplantation
diagnosis for the Kingsburys, said about half the embryos containing the
unwanted genetic profile were discarded and half donated to research.
‘Is This Genetic Engineering?’
Already, thousands of couples who are undergoing in vitro fertilization to
overcome infertility use P.G.D. to weed out embryos that harbor common
chromosomal disorders that would otherwise be screened for by amniocentesis.
Fertility experts say they may be the first to take advantage of the procedure
for a range of other genetic conditions.
Dr. Ina N. Cholst, a reproductive endocrinologist at Weill Medical College of
Cornell University, said a fertility patient of hers who suffers from an
inherited arthritic condition called ankylosing spondylitis was planning to add
genetic diagnosis to her in vitro procedure. She has a 50 percent chance of
passing the gene to a child. Of those who carry it, four of five will be
unaffected. The others will have arthritis, sometimes mild and sometimes quite
severe, but increasingly treatable.
“We brought it up,” said Dr. Cholst, who consulted with the patient’s
rheumatologist. “At the same time, I am thinking, ‘Is this a wonderful thing, or
is this genetic engineering?’ ”
In the future, many specialists believe, most in vitro fertilizations will be
performed for fertile couples seeking genetic diagnosis, not as a treatment for
infertility. But as it becomes easier to identify the possible consequences of
more kinds of genes, the decisions for parents may become harder. Having passed
over 4 embryos with the defective gene and identified 10 healthy ones, the
Kingsburys were asked if they wanted to pay $2,000 extra to test them for Down
syndrome. That test eliminated two more.
“You kind of feel like you shouldn’t be doing it,” Ms. Kingsbury said. “But then
why would we go through all of this and not take those extra precautions?”
Soon, experts say, prospective parents may be able to choose between an embryo
that could become a child with a lower risk of colon cancer who is likely to be
fat, or one who is likely to be thin but has a slightly elevated risk of
Alzheimer’s, or a boy likely to be short with low cholesterol but a significant
risk of Parkinson’s, or a girl likely to be tall with a moderate risk of
diabetes.
For the Kingsburys, the choice is still clear. Like any parents, they plan to
tell Chloe the story of her birth. And if all goes well, they say, she will soon
have a sibling who shares a similar tale.
Seeking Healthy
Children, Couples Cull Embryos, NYT, 3.9.2006,
http://www.nytimes.com/2006/09/03/health/03gene.web.html
Clarification Issued on Stem Cell Work
September 2, 2006
The New York Times
By NICHOLAS WADE
The scientific journal Nature has corrected a press release
and is considering whether to add further information to an article published
last week reporting that human embryonic stem cells could be generated without
destroying an embryo.
The article has political consequences because destruction of embryos, which is
unavoidable with present methods, is the main stated objection of many who
oppose embryonic stem cell research.
The journal’s action follows numerous news reports on the article and criticism
from the United States Conference of Catholic Bishops, which pointed out that
embryos used in the research were being destroyed, a fact made clear in the
scientific paper but not the press release.
Both the journal and the principal author of the article, Dr. Robert Lanza of
Advanced Cell Technology, say that the scientific report is correct and that any
addition would be solely for purposes of clarification.
“We’re looking to see if the description is clear, but there is nothing wrong
with the paper,” a Nature press officer, Ruth Francis, said yesterday.
For about 10 years, fertility clinics have sometimes used a genetic diagnostic
test in which a single cell is removed from an embryo at the eight-cell stage to
be tested for abnormalities like Down syndrome. If no defect is found, the
embryo with its remaining seven cells is implanted in the womb and grows to
term. Babies born from seven-cell embryos seem as healthy as others born after
in vitro fertilization.
Dr. Lanza noted in his article that the cell removed in this test, known as
preimplantation genetic diagnosis, or P.G.D., could be used, after growing and
dividing, both for testing and, with his new technique, to derive human
embryonic stem cells. Since the original embryo would be unharmed, a principal
objection to the research would be removed, he said.
Dr. Lanza’s article in Nature made clear that he had not saved the embryos in
his own experiments, in which he used as many as eight cells from each of some
16 donated embryos. Had he taken only a single cell from each, many more embryos
would have been needed. The press release issued by Nature, however, incorrectly
implied that he had removed just a single cell.
After news accounts based on the press release drew criticism from the bishops
conference, which opposes in vitro fertilization and human embryonic stem cell
research, Nature corrected its statement to make clear that Dr. Lanza’s embryos
had been destroyed. But the revised statement said other researchers, as he had
noted, could use his technique to derive stem cells from cells removed in P.G.D.
Dr. Lanza said yesterday that Nature had not shown him the original press
release before publication.
Clarification
Issued on Stem Cell Work, NYT, 2.9.2006,
http://www.nytimes.com/2006/09/02/science/02stem.html
|