|
History > 2009 > USA > Health (I)
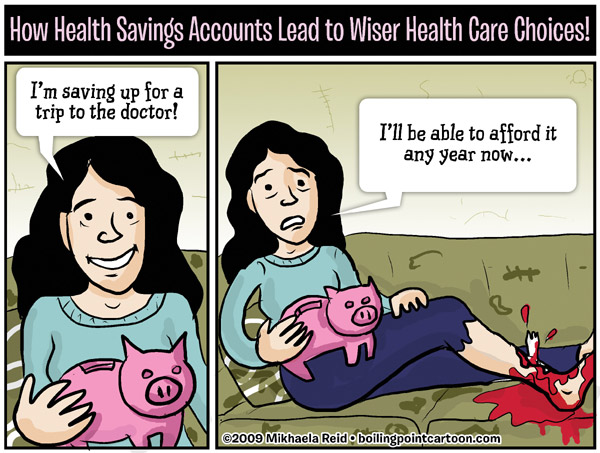
Mikhaela Reid
Cagle
16 January 2009
Op-Ed Contributor
Dead Body of Knowledge
March 27, 2009
The New York Times
By CHRISTINE MONTROSS
Providence, R.I.
AT the risk of sounding like a fuddy-duddy, I would like to say that
sometimes, medical imaging isn’t all it’s cracked up to be.
As a resident in psychiatry, I depend on the technology to treat my patients.
From countless computers in the hospital’s hallways and at nurses’ stations, I
call up images of the people I treat: the black, white and gray CT scans of
their skulls, the nuanced M.R.I.’s of their spinal cords and ligaments, the
rotating Spect scans that show in three dimensions how well — or how poorly —
blood flows through their brains. I can leave the room of an 89-year-old woman
who has begun picking imaginary bugs out of the air, look into a screen, and see
the tumor that is causing her delirium.
Now however, many medical schools are beginning to argue that imaging technology
has improved to the point where it should be used in place of the dissection of
human cadavers as the central tool of instruction for young doctors-to-be. This
is a mistake. No matter how detailed and versatile they become, computer images
can never provide the indelible lessons that novice doctors learn from real
bodies.
Nearly every medical student in America begins his career by entering a room
full of cadavers and taking one of them apart, layer by layer, piece by piece.
Doctors have shared this experience for centuries, ever since Vesalius, Da Vinci
and Michelangelo defied religion and government, stole bodies from graves and
churches, and dissected by candlelight in an audacious pursuit of knowledge
about the human body. The process is what you would expect: messy and smelly,
tedious and time-consuming, emotionally and physically difficult. It is at times
awe-inspiring, and at other times profoundly upsetting. It is also, for the
medical schools, very expensive. Even though cadavers are donated, it can cost
more than $2,000 to prepare a body for dissection.
So medical schools are beginning to re-evaluate their anatomy curriculum in the
face of the perhaps inevitable argument: Why not reduce, or eliminate
altogether, the burdensome cost of dissecting cadavers and replace it with this
new and astounding technology? The computers and software — a considerable
expense, but one that need be incurred only once — allow students to study
images of the body from every angle and on every plane. They can peel away the
muscle on a virtual leg to see the bone beneath, then click a different button,
reattach the muscle and see how the limb moves.
Computers can show things that still and lifeless cadavers cannot — blood
pumping in real time through the heart’s chambers, for instance. And it is far
easier to visualize nerves and vessels when they’re color-coded on a computer
than it is to pick through the indistinguishable gray-green tangles inside a
formalin-embalmed cadaver. Because all of this can be done anywhere on any
screen, students can study anatomy in this way in the library, in their
apartments or, surely someday if not already, on their iPods and cellphones.
At the end of the academic year, there would be no need for old cadavers to be
cremated, for new human donors to be found, for deep cleaning the anatomy lab.
Come September, the whole system would simply reboot.
But what kind of doctors will they be, these students who have never experienced
human dissection? They would have been denied a safe and more gradual initiation
into the emotional strain that doctoring demands.
Someday, they’ll need to keep their cool when a baby is lodged wrong in a
mother’s birth canal; when a bone breaks through a patient’s skin; when
someone’s face is burned beyond recognition. Doctors do have normal reactions to
these situations; the composure that we strive to keep under stressful
circumstances is not innate. It has to be learned. The discomfort of taking a
blade to a dead man’s skin helps doctors-in-training figure out how to cope,
without the risk of intruding on a live patient’s feelings — or worse, his
health. We learn to heal the living by first dismantling the dead.
The dissection of cadavers also gives young doctors an appreciation for the
wonders of the human body in a way that no virtual image can match. It is
awe-inspiring to hold a human heart in one’s hands, to appreciate its fragility,
intricacy and strength.
But most important, the cadavers on their stainless steel tables are symbols of
altruism to medical students: They are reminders of how great a gift one can
give to a stranger in the hopes of healing. Isn’t that the most fundamental
lesson we want our doctors to carry to the bedsides of their patients?
Christine Montross, a resident in psychiatry at Brown University, is the
author of “Body of Work: Meditations on Mortality From the Human Anatomy Lab.”
Dead Body of Knowledge,
NYT, 27.3.2009,
http://www.nytimes.com/2009/03/27/opinion/27montross.html
As New Lawyer,
Senator Defended Big Tobacco
March 27, 2009
The New York Times
By RAYMOND HERNANDEZ
and DAVID KOCIENIEWSKI
The Philip Morris Company did not like to talk about what went
on inside its lab in Cologne, Germany, where researchers secretly conducted
experiments exploring the effects of cigarette smoking.
So when the Justice Department tried to get its hands on that research in 1996
to prove that tobacco industry executives had lied about the dangers of smoking,
the company moved to fend off the effort with the help of a highly regarded
young lawyer named Kirsten Rutnik.
Ms. Rutnik, who now goes by her married name, Gillibrand, threw herself into the
work. She traveled to Germany at least twice, interviewing the lab’s top
scientists, whose research showed a connection between smoking and cancer but
was kept far from public view.
She helped contend with prosecution demands for evidence and monitored testimony
of witnesses before a grand jury, following up with strategy memos to Philip
Morris’s general counsel.
The industry beat back the federal perjury investigation, a significant legal
victory at the time, but not one that Ms. Gillibrand is eager to discuss.
Now in the Senate seat formerly held by Hillary Rodham Clinton, Ms. Gillibrand
plays down her work as a lawyer representing Philip Morris, saying she was a
junior associate with little control over the cases she was handed and limited
involvement in defending the tobacco maker.
But a review of thousands of documents and interviews with dozens of lawyers and
industry experts indicate that Ms. Gillibrand was involved in some of the most
sensitive matters related to the defense of the tobacco giant as it confronted
pivotal legal battles beginning in the mid-1990s.
Ms. Gillibrand, who worked at the Manhattan firm of Davis Polk & Wardwell from
1991 to 2000, eventually oversaw a team of associate lawyers working on Philip
Morris cases, according to a colleague, and was a frequent point of contact
between the firm and Philip Morris executives.
In addition, Ms. Gillibrand represented Davis Polk on a high-level Philip Morris
committee whose work included shielding certain documents from disclosure,
according to several lawyers and industry observers. Serving on the panel placed
her alongside some of the country’s top tobacco industry lawyers.
And she was viewed so positively by Philip Morris that by 1999, when the tobacco
maker brought in an additional outside law firm to represent its interests, Ms.
Gillibrand was one of five Davis Polk lawyers designated to train the firm about
sensitive legal issues, according to a company memo.
When she moved in 2001 to a new firm, Boies Schiller, where she worked until
2005, one of Ms. Gillibrand’s clients was the Altria Group, Philip Morris’s
parent company, where she helped with securities and antitrust matters,
according to the firm.
Ms. Gillibrand, 42, a former upstate congresswoman who is still unknown to many
New Yorkers and is preparing to defend her Senate seat in an election next year,
is reluctant to discuss her work on behalf of the tobacco company. After
initially agreeing to be interviewed by The New York Times, the senator canceled
through her spokesman, Matt Canter, who said that focusing on Philip Morris
would not reflect the range of her work as a lawyer, which also included
representing pro bono clients, including abused women and families contending
with lead paint in their homes..
“Senator Gillibrand was serving as a young associate when she was assigned this
case,” Mr. Canter said. “It is a small part of her 15-year legal career.”
He stressed that like other tobacco lawyers, she was not at liberty to discuss
her work for Philip Morris because of attorney-client privilege.
But those who recall Ms. Gillibrand’s days as a young lawyer say she was capable
and eager as she plunged into the high-stakes and lucrative world of tobacco
defense work.
“The client was always in her office,” said her former Davis Polk colleague
Vincent Chang, who spoke glowingly of Ms. Gillibrand. “She was probably accorded
more responsibility than the average associate by far.”
Of course, many lawyers, including some who now serve in the Senate, have
defended unpopular clients. Still, in an approach that was not uncommon at law
firms that represented tobacco companies, lawyers at Davis Polk were permitted
to decline work on the tobacco cases if they had a moral or ethical objection to
the work, Mr. Chang said.
Asked whether Ms. Gillibrand had any misgivings about representing the tobacco
company, Mr. Canter responded by e-mail: “Senator Gillibrand worked for the
clients that were assigned to her.”
Ms. Gillibrand was never the lead lawyer on the tobacco cases, which at Davis
Polk drew on the work of dozens of lawyers and staffers. Robert B. Fiske Jr., a
former Whitewater prosecutor and a Davis Polk partner, was the top lawyer among
the approximately 20 at the firm working on the Philip Morris defense on the
perjury case. Ms. Gillibrand’s hourly rate — $305 in 1995 — put her in the
middle range of reimbursement for associates on the case, according to a tobacco
industry document.
Mr. Fiske declined, through the senator’s office, to be interviewed about her
work for Philip Morris, but released a statement calling Ms. Gillibrand “smart,
hard-working and thoughtful.”
During her most recent congressional race, Ms. Gillibrand, who is a former
smoker, accepted $18,200 in campaign donations from tobacco companies and their
executives — putting her among the top dozen House Democrats for such
contributions. Many Congressional Democrats do not accept tobacco money.
Mr. Canter said the senator should be assessed based on her record in Congress,
where she has voted against the industry’s interests on several occasions,
including supporting cigarette tax increases to help expand children’s health
care.
And Todd Henderson, an assistant professor at the University of Chicago Law
School, argued that it would be unfair to assess lawyers by whom they represent.
“Nobody would want to live in a world in which lawyers are judged by the clients
they take,” he said.
Limiting Evidence
A scion of a prominent Albany political clan, Ms Gillibrand graduated from law
school at the University of California, Los Angeles, in 1991 and took a job at
Davis Polk, a firm that had worked closely with the tobacco industry for
decades.
Ms. Gillibrand was working at the firm during critical years for the tobacco
industry, as the public tide was turning against smoking, and leading Democrats
in the Clinton administration and Congress pushed for a more aggressive stance
toward cigarette companies. At the same time, plaintiffs’ lawyers were beginning
to chip away at the industry’s time-tested legal strategies.
In 1994, executives of the nation’s largest tobacco companies, including Philip
Morris, prompted anger and disbelief when they swore before Congress that they
did not believe smoking was addictive or that there was a proven link between
smoking and cancer.
That appearance intensified criticism of the industry and scrutiny by federal
prosecutors and ultimately led to a broad criminal investigation by the Justice
Department into whether the executives had perjured themselves.
The government sought reams of internal company records to determine whether the
tobacco executives had lied. There is no indication that Ms. Gillibrand ever
discussed the case with William Campbell, then the Philip Morris president and
chief executive, who was among the subjects of the perjury inquiry. But Philip
Morris internal records show that the company’s top lawyers entrusted her with
several essential elements of the case.
As a member of the Eastern District of New York Subpoena Working Group, Ms.
Gillibrand helped limit what evidence the government obtained. She also
monitored the testimony of witnesses who appeared before the grand jury and
wrote strategy memos to the Philip Morris general counsel, Ken Handal, analyzing
the witnesses’ statements and their impact on the investigation.
Her travels to Germany took her to the Institut Fur Biologische Forschung, or
Institute for Biological Research, a laboratory that Philip Morris had set up in
Cologne, which has been criticized by antitobacco activists and cancer doctors.
The establishment of the lab overseas, where topics of study included the role
of tobacco in cancerous tumors, had allowed the company to keep conducting
research there, beyond the reach of the United States government, news media and
plaintiffs’ lawyers.
Ms. Gillibrand learned so much about the laboratory’s inner workings during the
criminal investigation that by 1997, records show, she provided Philip Morris
lawyers with a list of questions about the German lab to help them prepare
company witnesses being called to testify in civil cases in Minnesota and
elsewhere across the country.
At the laboratory, she interviewed Dr. Max Reininghaus, the general manager who
oversaw the experiments, and reviewed lab personnel records that had been sought
by federal investigators.
In 1998, when the case reached a turning point as one tobacco company, the
Liggett Group, considered cooperating with prosecutors, Ms. Gillibrand was one
of a handful of lawyers for Philip Morris privy to the unsuccessful efforts to
dissuade Liggett from breaking ranks with the other cigarette makers.
She was also among the small group of Philip Morris lawyers involved in the
effort to contain the damage the defection could do to other companies in the
tobacco industry, pushing to prevent Philip Morris from disclosing any documents
that would violate the confidentiality of the other co-defendants.
“She clearly was more than a lowly associate lawyer on the case,” said Anne
Landman, a tobacco document researcher who has testified against the industry
and edits Tobaccowiki.org, a Web site that provides analysis of tobacco
documents. “Philip Morris showed deep trust in her and brought her in on
sensitive legal matters that were of great importance to the company.”
In the face of the vigorous counteroffensive from the industry, the Justice
Department abandoned its criminal inquiry in 1999 and decided to bring a
racketeering case in civil court, claiming that the cigarette companies
conspired for half a century to mislead the public about the dangers of smoking.
Ms. Gillibrand did not work on the racketeering case, on which other law firms
took the lead. But when Judge Gladys Kessler of Federal District Court handed
down her landmark decision in that case in 2006, finding that the tobacco
companies had conspired to defraud the public, she based the ruling in part on
the business practices Ms. Gillibrand had delved into during the perjury case.
The judge cited Philip Morris’s use of the German lab as a way for the company
to suppress evidence and scolded the company for concealing information from
consumers and government regulators.
Asked last week whether Ms. Gillibrand agreed with the judge’s decision, her
spokesman replied: “Senator Gillibrand did not work on that case and is not
familiar with its details.”
A Rising Star
Ms. Gillibrand was also deeply involved as Philip Morris and other cigarette
makers confronted another challenge: mounting accusations that the industry was
abusing the attorney-client privilege to prevent disclosure of damaging research
and other sensitive documents.
Legal experts and a Congressional committee said that for decades, the companies
had misused the attorney-client privilege to try to conceal scientific
information that was damaging to the industry. The lawyers, for example,
participated in overseeing scientific research projects that they could then
keep confidential. But in the 1990s, government and plaintiffs’ lawyers began
directly challenging this protection.
The state of Minnesota, as part of a lawsuit seeking to force tobacco firms to
pick up the state’s cost of treating smoking-related illnesses, objected to the
companies’ claim of attorney-client privilege, invoking what is known as the
crime-fraud exception: essentially, an assertion that the privilege did not
apply because the lawyers were being used to help the companies commit fraud. A
Minnesota judge agreed, saying that Philip Morris had engaged in an “egregious
attempt to hide information” and, in a major blow to the industry, eventually
forced the release of some 30 million pages of documents from industry files.
Philip Morris and the other companies subsequently settled the Minnesota case
for $6 billion in 1998.
But with the industry facing other lawsuits around the country, Philip Morris
turned to a committee it established to handle issues surrounding disclosure of
other documents. In some instances, the committee sought to determine if certain
documents had been improperly shielded under attorney-client privilege rule. But
the committee also worked to protect other industry documents from being
released, a practice that drew harsh criticism from lawyers and others who took
on the industry.
Clifford Douglas, who served as a lawyer for the Congressional task force that
looked into the tobacco industry’s practices, said, “The crime fraud committee
was charged with preventing plaintiffs or the government from seeing sensitive
documents that Philip Morris wanted to keep secret.”
Some of the nation’s most prominent tobacco lawyers from several prestigious law
firms had seats on the committee, known as the Philip Morris Crime Fraud Issues
Committee. And so did Ms. Gillibrand, who was already seen as a rising star
among her colleagues at Davis Polk.
Mr. Chang, who worked with her at the firm, said it was telling that Ms.
Gillibrand would be assigned to the panel along with “the linchpins of the
tobacco defense bar in the entire country.”
“That’s certainly an indicator of the kind of respect that she was accorded at
Davis Polk that they would chose her — a relatively junior associate — to be on
a panel with some of the most prominent senior tobacco lawyers in the country,”
he said.
Leslie Wharton, a senior counsel at the Washington law firm of Arnold Porter
L.L.P. and a member of the crime fraud committee, said that although Ms.
Gillibrand had less experience and stature than other lawyers on the panel, she
was assertive, deeply involved and very effective in advocating on behalf of
Philip Morris.
“She did more than pull her own weight,” Ms. Wharton said. “We handled highly
specialized issues on a whole variety of cases, and she was a full partner in
everything we did. She worked as hard as anyone and was a very capable, smart
lawyer.”
Much of the committee’s work remains sealed, but internal documents indicate
that the committee had wide latitude and “should be consulted with respect to
just about any privilege issue that might arise in any case.”
A Philip Morris spokesman declined to discuss the committee or when it was
formed.
Helping With Strategy
At Davis Polk, lawyers not only represented Philip Morris in litigation, they
advised the company on business strategy, including how to protect the image of
the cigarette company and how to deal with concerns about the effects of its
products. This approach reflects, in part, the longstanding closeness between
the firm and tobacco makers. But it also raised concerns among critics that the
lawyers had crossed a line, and were essentially becoming agents in the business
operation.
There were instances, for example, when Ms. Gillibrand was called upon to help
the company deal with mounting public unease about its product and practices,
according to interviews and a review of industry documents. Ms. Gillibrand was
also schooled in some of the chemistry of cigarettes.
In 1998, for example, Roger G. Whidden, Philip Morris’s vice president for
worldwide regulatory affairs, wrote Ms. Gillibrand a letter along with a draft
document containing proposed responses to possible questions from reporters
about nitrosamines, a cancer-causing agent in cigarettes.
In the letter, Mr. Whidden tells Ms. Gillibrand that the draft was prepared “on
the basis of conversations” with her and others at Philip Morris, and asks her
to review it. The suggested answers state that Philip Morris is working to
reduce the presence of the deadly agent in cigarette smoke.
But the document also makes an assertion that experts say is highly misleading.
The document declares flatly that the amount of nitrosamines in cigarette smoke
had been reduced through filtration. That assertion was not in keeping with what
was known about limitations of certain cigarette filters at the time, the
experts say: smokers frequently compensated for them by inhaling more deeply,
plugging up filter ventilation holes with their fingers or lips or taking more
puffs.
The tobacco companies had been aware of this flaw in the filters for decades,
according to industry documents and interviews, and Ms Gillibrand had just weeks
before been briefed on their shortcomings and had taken a tour of the filtration
section of Philip Morris’s production plant, according to company documents.
The presentation was given by Bill Dwyer, a scientist in the company’s research
and development division, who described, among other things, how plugging the
ventilation holes of filters diminishes the effectiveness of the filters.
As New Lawyer,
Senator Defended Big Tobacco, NYT, 27.3.2009,
http://www.nytimes.com/2009/03/27/nyregion/27gillibrand.html?hp
Contraception Pill Strictures
Are Eased by a Judge
March 24, 2009
The New York Times
By NATASHA SINGER
A federal judge ordered the Food and Drug Administration on
Monday to make the Plan B morning-after birth control pill available without
prescription to women as young as 17.
The judge ruled that the agency had improperly bowed to political pressure from
the Bush administration in 2006 when it set 18 as the age limit.
The agency has 30 days to comply with the order, in which the judge also urged
the agency to consider removing all restrictions on over-the-counter sales of
Plan B. The drug consists of two pills that prevent conception if taken within
72 hours of sexual intercourse.
Some women’s health advocates hailed the decision.
“It is a complete vindication of the argument that reproductive rights advocates
have been making for years, that in the Bush administration it was politics, not
science, driving decisions around women’s health,” said Nancy Northup, president
of the Center for Reproductive Rights, the attorneys for the plaintiff in the
suit against the F.D.A.
But some conservative groups voiced concern that the ruling could promote sexual
promiscuity. “Now some minor girls will be able to obtain this drug without any
guidance from a doctor and without any parental supervision,” the Family
Research Council said in a statement.
Plan B has been available by prescription in the United States since 1999.
But because the drug must be taken so soon after intercourse to be effective, in
2001 more than five dozen public health groups, with endorsements from World
Health Organization and the American Medical Association, asked the F.D.A. to
make Plan B available over the counter.
Not until 2006 did the F.D.A. rule, saying that the drug could be sold without a
prescription only to women over 18. In order to enforce the age restriction, the
agency also ordered that Plan B be stocked behind pharmacy counters, in contrast
to other over-the-counter contraceptives like condoms.
On Monday, in a decision that criticized former F.D.A. officials, Judge Edward
R. Korman of Federal District Court in New York threw out the F.D.A. ruling.
Judge Korman wrote that officials of the agency had repeatedly delayed action on
the petition, moving only when members of Congress threatened to hold up
confirmation hearings on acting F.D.A. commissioners. Several officials also
violated the agency’s own policies, he wrote.
Citing depositions, Judge Korman wrote that agency officials had improperly
communicated with White House officials about Plan B. And, he said, F.D.A.
employees sought to influence decisions by appointing people with anti-abortion
views to an independent panel of experts reviewing Plan B for the agency.
The agency also departed from its normal procedures, the judge wrote, by
ignoring favorable conclusions about the drug by an advisory panel as well its
own scientists and officials who found that the drug could be safely used by
women at least as young as 17.
Such “political considerations, delays and implausible justifications” showed
that the F.D.A. had acted without good faith or reasoned decision making, Judge
Korman wrote.
Susan F. Wood, a former F.D.A. director of women’s health who resigned in 2005
to protest the handling of Plan B, said Monday that the judge’s decision to send
the drug back for reconsideration signaled hope of the agency’s ability to act
independently under a new administration.
There is a new chance to “restore the scientific integrity of the F.D.A.,” said
Ms. Wood, now a professor of public health at George Washington University.
In response to a query from a reporter, an F.D.A. spokeswoman wrote Monday in an
e-mail message that the agency was still reviewing the decision.
Contraception Pill
Strictures Are Eased by a Judge, NYT, 24.3.2009,
http://www.nytimes.com/2009/03/24/health/24pill.html?hp
Editorial
The Rules on Stem Cells
March 16, 2009
The New York Times
No sooner had President Obama lifted the Bush-era restrictions
on financing embryonic stem cell research than critics began urging that any
federal support be limited to work with stem cells derived from surplus embryos
at fertility clinics. That would be a mistake. The guidelines should define the
eligible research as broadly as possible to allow the greatest potential for
advances.
Some of the most important research requires stem cells genetically matched to
patients with specific diseases, such as Parkinson’s or diabetes. These can
rarely be identified in the huge stock of surplus embryos.
President Bush limited scientists to 21 stem cell lines derived from surplus
embryos before mid-2001. Congress twice passed bills to allow potentially
thousands more lines from surplus embryos to be used. Mr. Bush vetoed them.
(Hundreds of stem cell lines have been created around the world, all or
virtually all from surplus embryos.)
This single-minded focus on the surplus embryos — left over after patients’
fertility treatments were completed — was mostly because a strong moral argument
could be made that these microscopic, days-old embryos were doomed to be
discarded anyway. Why not gain potential medical benefits from studying their
stem cells?
Now President Obama seems open to the possibility of moving beyond the surplus
embryos. His announcement placed few boundaries on stem cell research beyond
requiring it to be scientifically worthy, responsibly conducted and compliant
with the law.
He gave the National Institutes of Health free rein to devise guidelines
governing what kinds of research can be supported and what ethical strictures
will be placed on it.
Let us hope that the N.I.H. broadens the range of stem cells that can be
studied.
Scientists believe that one way to obtain the matched cells needed to study
diseases is to use a cell from an adult afflicted with that disease to create a
genetically matched embryo and extract its stem cells. This approach — known as
somatic cell nuclear transfer — is difficult, and no one has yet done it.
Another approach — known as induced pluripotent stem cells — has shown that
adult skin cells can be converted back to a state resembling embryonic stem
cells without ever creating or destroying an embryo. Some experts think that
approach may be the most promising, for moral and practical reasons.
Even so, work on genetically matched embryonic stem cells would still be
important. They may be the best way to study the earliest stages of a disease,
or prove superior for other purposes. They will almost certainly be needed as a
standard to judge the value of the induced pluripotent cells.
When the N.I.H. sets the rules for federally financed research, the main
criterion should be whether a proposal has high scientific merit.
The Rules on Stem
Cells, NYT, 16.3.2009,
http://www.nytimes.com/2009/03/16/opinion/16mon1.html
Letters
Doctor-Patient-Computer Relationships
March 11, 2009
The New York Times
To the Editor:
Re “The Computer Will See You Now”
(Op-Ed, March 6):
Dr. Anne Armstrong-Coben has provided a spot-on description of the fallibilities
of the electronic medical record from the standpoint of the doctor-patient
encounter. It can depersonalize situations that require personal interaction. To
her account, let me add one thing I’ve noticed.
All too often when taking a history, residents and attendings in a hurry will
simply use the cut-and-paste function to save time and bypass asking potentially
important questions that have been asked before. Without ever undergoing
independent verification, erroneous data from the original history gets
perpetuated. Doctors rely on others for their own versions of events, a
potential source of misinformation and error.
Cory Franklin
Wilmette, Ill., March 6, 2009
The writer is a former director of medical intensive care, Cook County Hospital,
Chicago.
•
To the Editor:
Dr. Anne Armstrong-Coben presents her experience in adapting to electronic
medical records as a cautionary tale, proscribing an open embrace of the
computer in the doctor-patient relationship.
Is it too much to expect that physicians who continually educate themselves on
the latest medical treatments also integrate the latest improvements in
delivering that care? I have found that my last year with electronic medical
records in my practice has been a step forward for the health of my patients.
Maintaining the doctor-patient relationship required adaptation to technology,
not submission to it.
Jihad Shoshara
La Grange, Ill., March 6, 2009
The writer is a medical doctor.
•
To the Editor:
I must agree wholeheartedly with Dr. Anne Armstrong-Coben: the presence of a
computer in the exam room is more of a detriment than an advantage.
The physician focuses on the computer and misses patient or parental body
language (I’m a pediatrician, too). Awful mistakes may get clicked and pass
unnoticed. Girls may be described as having male genitalia and vice versa.
What is most troublesome to me is that our residents — no matter how much we
grayheads or graybeards model the approach to history-taking from parents —
learn that the checklist on the computer screen is the thing to look at, and not
the living person describing their child and her problems.
Howard Fischer
Detroit, March 6, 2009
The writer is a professor of pediatrics at Wayne State University School of
Medicine.
•
To the Editor:
I could not agree more with Dr. Anne Armstrong-Coben. The computers we all use
were invented as bookkeeping machines. At this they are superb.
They are not good at shoehorning the analog world into digital format. In fact,
computers aren’t meant to help physicians; they are meant to gather data for
others at the expense of physicians’ time, attention and job performance.
I am an anesthesiologist. In the operating room, computerization is useful for
recording metrics like pulse and blood pressure. Even here, computers hijack my
attention and interfere with my job.
We can’t do two tasks at once, and computers require just that.
Michael Cox
Santa Barbara, Calif., March 6, 2009
•
To the Editor:
A mentor of mine once told a story of a patient whose son admired a certain
Russian poet. He made a notation of this in his office note.
Several years later, upon seeing this woman again, he inquired as to whether her
son “still read Pushkin.” The patient nearly fell off her chair, so taken was
she by her doctor’s recalling this seemingly trivial but meaningful fact.
I have used this anecdote repeatedly in my own career, jotting down reminders as
to my own patients’ reading proclivities and other personal miscellany. Bringing
up these tidbits at future visits is useful in creating trust between patient
and doctor.
Unfortunately, I have yet to encounter an electronic medical record system that
allows one to document the reading habits of the patient or his family members.
Ronald B. Cohen
Woodbury, N.Y., March 6, 2009
•
To the Editor:
Dr. Anne Armstrong-Coben makes a legitimate point about computerizing
physicians’ notes. The model she describes is simply a glorified questionnaire,
similar to the one on that clipboard we’re asked to fill out in the waiting room
during our first visit. That model is neither efficient nor effective.
What could work would be to take notes as she is now doing, then scan them into
the permanent electronic record. This would achieve the accessibility of
electronic records, and the personalization she wishes to retain.
As for the illegible handwriting: That’s a product of laziness; almost anyone
can teach himself to write legibly!
Warren Bailey
Qualicum Beach, British Columbia
March 6, 2009
The writer is a retired cardiac surgeon.
•
To the Editor:
This transference from human to computer, as if the records become the human,
isn’t special to the medical profession. It exists in other professions and in
any institution where personal data are electronically gathered.
It explains how you can feel as if you barely exist when in front of a keeper of
these records, whether a doctor or a clerk at your local motor vehicle agency.
As they stare intently into their computer screens, they are looking at the real
you that exists for them, the one in the computer.
Peter Carr
Berkeley, Calif., March 6, 2009
•
To the Editor:
Dr. Anne Armstrong-Coben’s story is a cautionary tale. It illustrates why most
of the $17 billion in President Obama’s stimulus package for promoting
electronic medical records will add to the cost of care, not reduce it.
Dr. Armstrong-Coben is applying the computer to a method of pediatric practice
that has been in effect from time immemorial. The cost of computers has been
added to the cost of her practice without, it seems, improving her efficiency.
What she ought to be doing is thinking up a new, better and less expensive way
to provide health care to children that is practical only with computers and
then get someone to develop a program to support it.
Not being a pediatrician, I would not presume to suggest what that way might be,
but I’m sure that it is out there waiting to be discovered.
Richard Wittrup
Scituate, Mass., March 6, 2009
The writer is a retired hospital administrator.
Doctor-Patient-Computer Relationships, NYT, 11.3.2009,
http://www.nytimes.com/2009/03/11/opinion/l11medical.html
21% of Americans
scramble to pay medical, drug bills
10 March 2009
USA Today
By Liz Szabo and Julie Appleby
Denise Prosser, 39, has battled cancer since she was a
toddler.
Yet Prosser can't afford her next cancer treatment — a
radioactive therapy that she's supposed to receive once a year — because she and
her husband lost their jobs in December. Without insurance, she has postponed
the radiation indefinitely and is taking only half of her asthma medications —
sacrifices that often leave her gasping for air and could allow her cancer to
come surging back.
"I can't walk more than 100 feet without sounding like I just
ran a marathon," says Prosser, of Galloway, N.J.
Prosser is among millions of Americans who struggled last year to pay for health
care or medications, the largest poll ever conducted by Gallup shows.
As the economy fell, the percentage who reported having trouble paying for
needed health care or medicines during the previous 12 months rose from 18% in
January 2008 to 21% in December, according to the poll of 355,334 Americans.
Each percentage point change in the full survey represents about 2.2 million
people, says Jim Harter, Gallup's chief scientist for well-being and workplace
management.
Gallup, along with disease management company Healthways, surveyed a random
sample of about 1,000 people nearly every day during 2008 about their physical,
emotional and economic well-being.
The poll, the Gallup-Healthways Well-Being Index, shows that struggles to pay
crossed all socioeconomic lines but hit some Americans harder than others: More
than half of the uninsured had trouble paying for health care or medications
during the year. So did more than 30% of blacks and Hispanics, compared with 17%
of whites and 13% of Asians. Overall, women had more trouble than men. Those who
were divorced, widowed or in domestic partner arrangements fared less well than
those who were married.
Among other key findings:
• As the year progressed, fewer Americans reported getting health coverage
through their jobs, dropping from 59% in the first quarter to 58% by the last.
• The number of African Americans reporting trouble paying for health care or
medications rose six percentage points from the first quarter to the last, to
34%. People ages 25-34 also saw a big increase, up five points to 28%.
• Among the states, Hawaii had the smallest percentage of residents who had
trouble paying for health care in the previous 12 months at 12%, and Mississippi
the most at 29%.
"The biggest problem that the country has is actually the cost of health care,"
says Jim Clifton, Gallup's CEO. "It's a lot bigger problem than war and a bigger
problem than the current meltdown because there are no fixes to it on the
horizon right now. … You can't just throw money at it. That's still not a fix."
The increasing trouble people have paying for medical care comes as Congress
begins its most serious health care overhaul debate in 15 years — and as the
economy continues to shed jobs.
Because most people still get health insurance through their jobs — rather than
buying it themselves or being covered by a government program such as Medicare —
the loss of a job can mean the loss of insurance.
Nearly 4.4 million people have lost jobs since the recession began in December
2007, the U.S. Department of Labor reports. Nearly one in 10 children and one in
five adults under age 65 are uninsured, says a February report on the uninsured
from the Institute of Medicine, part of the National Academy of Sciences, which
advises the government on health care.
People without insurance are at much higher risk for a host of medical problems,
the institute's report shows. They're less likely to get preventive care, more
likely to be diagnosed with later-stage cancers and more likely to die if they
suffer a heart attack, stroke, lung problem, hip fracture, seizure or trauma.
"The evidence clearly shows that lack of health insurance is hazardous to one's
health," says report co-author Lawrence Lewin. "And the situation is getting
worse."
Lower-income residents are more likely to have trouble paying medical bills and
to lack insurance. Income also plays a role in how people feel about their own
physical well-being.
The Gallup-Healthways poll found that 40% of those making $500 to $1,000 a month
said they were dissatisfied with their health. By comparison, only 10% of
wealthy people — those making at least $10,000 a month — are dissatisfied with
their health.
Few safety nets
People often resort to desperate solutions to pay for health care for themselves
and their families, says Christy Schmidt, senior policy director at the American
Cancer Society's Cancer Action Network.
Some are tapping into their 401(k) plans and other retirement savings, she says.
But even these funds may fall short, since many investments have lost half their
value in the past year.
When money gets really tight, Schmidt says, many uninsured people cut corners on
their health, such as by cutting pills in half or skipping doctor's
appointments.
While Gallup's poll asked if the specific person being interviewed had cut back
on "needed" health care, a February poll by Kaiser Family Foundation took a
broader look at health care spending. In that poll, more than half of Americans
said at least one person in their family had cut back on medical care within the
previous 12 months because of cost.
Many people can't pay for coverage on their own, Schmidt says.
Among them are Denise Prosser, who worked part time in a day care before being
laid off, and her husband, Warren, who was a television news director in
Linwood, N.J.
The 600 stitches on her back testify to her long struggle with cancer. She was
first diagnosed at 18 months old. A new tumor, in her thyroid, developed when
she was 27. Her lung capacity has declined by 50% since then as her health has
deteriorated, leaving her unable to work full time.
The Prossers say they can't afford coverage through COBRA, a program that allows
workers to keep their health insurance for 18 months after they leave their
jobs, just as long as they pay 100% of the health premiums themselves.
A COBRA plan would cost the Prossers $900 a month, Denise says. With help from
the recently passed economic stimulus package, which provides a federal subsidy
worth 65% of COBRA premiums, the Prossers still would have to pay $300 a month —
an especially high price tag for people who no longer have regular salaries.
After she lost her job, Prosser applied for official status as disabled through
the Social Security Administration but was turned down: "They said I wasn't
disabled enough."
Even patients who qualify as disabled may struggle with medical bills, Schmidt
says. Most people have to wait two years after being declared disabled before
they qualify for Medicare coverage. If patients opt for 18 months of COBRA, that
still leaves a six-month gap.
That puts Prosser — whose doctor recently found a lump on her thyroid — in a
sort of no man's land.
Prosser fears the lump could be a relapse of the thyroid cancer she developed in
1997. Although her thyroid specialist gave her some free medication samples, the
doctor would not treat Prosser without insurance.
Prosser hopes to see a doctor through a charity clinic in Atlantic City but
worries her husband's income from his unemployment check — $622 a week before
taxes — may disqualify them.
A domino effect
Even charity care and emergency rooms can't guarantee that uninsured people —
especially those such as Prosser, who have a long history of complex problems —
get the treatment they need, says John Ayanian, a Harvard Medical School
professor and co-author of the Institute of Medicine report. Free clinics often
struggle just to find generalists, he says, let alone specialists.
The problem extends beyond individual struggles.
Eroding insurance coverage can undermine the health of entire communities,
Ayanian says. Hospitals and doctors may have trouble paying their own bills in
communities with large numbers of uninsured. That can drive away specialists and
make it harder for even well-insured people to find care, the report says.
Often, people without insurance must struggle on their own.
Calls to the cancer society's insurance hotline have increased by 6% since last
year, Schmidt says. Although the society sometimes can help patients find
coverage, three out of five callers find those options — such as individual
health policies or state-sponsored high-risk pools — too expensive, Schmidt
says.
Nor is there any guarantee those options will be available. Individual policies
sometimes won't cover pre-existing medical conditions, such as cancer,
depression or pregnancy, or will not pay for care needed for those conditions
during an initial period of six months or more.
Dropping insurance
Jim Hann, 51, who's losing his job as a chemical operator at the Americas
Styrenics plant in Marietta, Ohio, next month, won't be able to afford COBRA,
even with the federal subsidy. The plant is laying off 65 of 100 employees. That
didn't deter him, however, from donating a kidney to his wife, Hannah.
In the past decade, Hannah has weathered more surgeries than
they can count: seven or eight operations to cut away dying sections of bowel, a
small intestine transplant and, in February, the kidney transplant at
Washington's Georgetown University Hospital.
"He tells me he'd give me both of his if that's what it took," says Hannah, 49,
a few days after the February transplant.
Their surgeon moved up her transplant surgery by a month, before Jim's coverage
lapsed. Although Hannah's disability makes her eligible for Medicare, she has
used Jim's generous company-funded insurance until now. Medicare will cover her
health care after Jim loses his coverage in November.
Jim plans to get by without any insurance. That's a gamble, given that kidney
donors have an increased risk of high blood pressure and kidney problems.
After taking care of Hannah for so many years, Jim says he's well-prepared for
his next career.
He has decided to enroll in a nursing program that will make him a registered
nurse within two years. Until then, he says, the couple will "tough it out" by
living off their savings, Hannah's disability check and the proceeds they make
selling their home to move into a smaller, cheaper house. If needed, Jim says
he's prepared to return to driving a truck or waiting tables while going to
school.
But he doubts he'll ever find another job like the one he lost. Factories are
laying off at least 1,000 workers in the region around Marietta and Ravenswood,
W.Va., about 50 miles away, where Century Aluminum is shutting down a plant and
letting go about 600 employees.
The Gallup-Healthways survey found nearly 25% of people in the congressional
district that includes Marietta didn't have enough money to pay for health care
in the past year.
"There aren't even any bad jobs," Jim says. "It's the same all over."
Contributing: Susan Page
21% of Americans
scramble to pay medical, drug bills, UT, 10.3.2009,
http://www.usatoday.com/news/health/2009-03-10-gallup-medical-bills_N.htm
Editorial
Science and Stem Cells
March 10, 2009
The New York Times
We welcome President Obama’s decision to lift the Bush
administration’s restrictions on federal financing for embryonic stem cell
research. His move ends a long, bleak period in which the moral objections of
religious conservatives were allowed to constrain the progress of a medically
important science.
Even with this enlightened stance, some promising stem cell research will still
be denied federal dollars. For that to change, Congress must lift a separate ban
that it has imposed every year since the mid-1990s.
Mr. Obama also pledged on Monday to base his administration’s policy decisions
on sound science, undistorted by politics or ideology. He ordered his science
office to develop a plan for all government agencies to achieve that goal.
Such a pledge should be unnecessary. Unfortunately, for eight years, former
President George W. Bush did just the opposite. He chose scientific advisory
committees based on ideology rather than expertise. His political appointees
aggressively ignored, distorted or suppressed scientific findings to promote a
political agenda or curry favor with big business.
This cynical approach seriously hampered government efforts to address global
warming and encourage sound family planning practices, among other issues.
President Obama was appropriately cautious, warning that the full promise of
stem cell research remains unknown and should not be overstated. Some of the
benefits, he said, might not appear in our lifetime or even our children’s
lifetime. But scientists hope that stem cell therapies may eventually lead to
treatments or cures for a wide range of degenerative diseases, such as
Parkinson’s and diabetes, and Mr. Obama rightly promised to pursue the research
with urgency.
In one of his first acts as president, Mr. Bush restricted federal financing for
embryonic stem cell research to what turned out to be 20 or so stem cell lines
that had been created prior to his announcement. Those lines are too limited in
number, variety and quality to allow the full range of needed research.
With the end of the Bush restrictions, scientists receiving federal money will
be able to work with hundreds of stem cell lines that have since been created —
and many more that will be created in the future. The full range of additional
research allowed won’t become apparent until new guidelines governing what
research can qualify for federal support are issued by the National Institutes
of Health.
Other important embryonic research is still being hobbled by the so-called
Dickey-Wicker amendment. The amendment, which is regularly attached to
appropriations bills for the Department of Health and Human Services, prohibits
the use of federal funds to support scientific work that involves the
destruction of human embryos (as happens when stem cells are extracted) or the
creation of embryos for research purposes.
Until that changes, scientists who want to create embryos — and extract stem
cells — matched to patients with specific diseases will have to rely on private
or state support. Such research is one promising way to learn how the diseases
develop and devise the best treatments. Congress should follow Mr. Obama’s lead
and lift this prohibition so such important work can benefit from an infusion of
federal dollars.
Science and Stem
Cells, 10.3.2009,
http://www.nytimes.com/2009/03/10/opinion/10tue1.html
Drug Investors Lose Patience
March 10, 2009
The New York Times
By ANDREW POLLACK
As merger mania plays out among the pharmaceutical giants, a different sort
of financial frenzy has seized some small, struggling drug makers. Investors are
demanding that stragglers close up shop and hand over any remaining cash.
That is what happened to one company, Avigen, after its most promising drug
failed in a clinical trial last October. Avigen said it would do what countless
other biotechnology companies had done in similar circumstances: move on to the
next product in its pipeline.
Not so fast, said its biggest shareholder, the Biotechnology Value Fund. The
fund demanded that Avigen, after 16 years of trial and error, immediately
liquidate itself and return its remaining cash to shareholders.
So much for the traditional model of patience in biotechnology investing, in
which companies may burn through more than a decade and hundreds of millions of
venture capital or shareholder dollars before reaching profitability — if they
ever get there. Now, with cash scarce, credit tight and big drug companies like
Merck intent on branching into biotechnology themselves, struggling start-ups
may no longer get second and third chances to succeed.
In at least eight cases in the last year, anxious investors have tried to block
an unsuccessful biotech company’s quest for the next blockbuster, and have
fought with management for control of the corporate carcass. The investors argue
that the remaining cash belongs to them and that they — not a losing company’s
executives — should decide how to invest it.
Some companies, including Avigen, are fighting back. “I hear that argument”
about shareholder rights, said Kenneth G. Chahine, Avigen’s chief. “But it’s
really ‘I want to raid the cash.’ We’re back to 1987 and ‘Barbarians at the
Gate.’ ”
Such battles have become much more common in recent months, as the stock market
crash has pounded the value of many biotech companies to less than the cash on
hand. When that happens, investors can realize an immediate return if the
company dissolves itself — even if some of the cash will be consumed in closing
the company.
In some cases, investors are succeeding. Under pressure from the hedge fund RA
Capital Management, for example, Northstar Neuroscience, a medical device
company in Seattle whose stroke treatment failed, is proposing to liquidate,
with shareholders receiving an estimated $1.90 to $2.10 a share in cash. The
company’s stock, which had been as low as 90 cents in November, closed at $1.90
on Monday.
Another company, Trimeris, whose only product, the AIDS drug Fuzeon, has lost
sales to newer competitors, halted research and development last year and repaid
$55 million — or $2.50 a share — to stockholders. The company continues in
business, but with few employees.
And two companies, VaxGen and NitroMed, have canceled planned reverse mergers
because of shareholder opposition. In a reverse merger, a publicly traded
company essentially cedes its cash and stock listing to a private company with
presumably better prospects.
For every Gilead Sciences, which spent $450 million over 15 years and abandoned
its original technology before becoming profitable, there have been countless
“zombies” — companies that lurch from product to product, surviving years or
even decades without ever achieving success.
One company so tarred, by one of its biggest investors, is Penwest
Pharmaceuticals.
“The company’s history is an unfortunate progression of failed development
programs,” Perceptive Advisors, an investor in the company, wrote in November to
Penwest’s board. Perceptive demanded that Penwest cease all research and
development and become a virtual company that would just collect royalties on
its one successful drug. Penwest defended its track record and said it was
sticking to its course.
Some investors say that with capital markets now so tight, the walking dead
should be buried to free up financing for more viable companies. “It’s in a time
like this that the good companies are being dragged down by the bad ones,” said
Oleg Nodelman, a portfolio manager at the Biotechnology Value Fund.
In some cases, however, the investors asking for their money back are not
long-suffering shareholders. They are speculators who bought in only after the
stock price collapsed, hoping to make a quick killing.
Tang Capital Partners, for instance, began accumulating its 14.9 percent stake
in Vanda Pharmaceuticals only after the Food and Drug Administration rejected
Vanda’s schizophrenia drug in July. Tang is now pressing for the company to
cease all operations and return cash to shareholders. Vanda’s stock is trading
at 80 cents, well below the $1.74 a share in cash it had as of Dec. 31.
Vanda says that it is still hopeful that it can get its drug approved and that
liquidation is not in the interest of all shareholders.
The Biotechnology Value Fund, often called BVF, was a longtime shareholder in
Avigen. But it sold 640,000 shares, nearly all its holdings, for about $3.95 to
$4.60 a share. The sale was near the stock’s highs for the year — in the two
months before Avigen was scheduled to announce, in October, the clinical trial
results of its drug to treat a symptom of multiple sclerosis.
After the drug failed, BVF swooped in and bought more than eight million shares,
nearly a 30 percent stake, at about 58 cents a share. That was well below
Avigen’s cash total of about $1.90 a share at the time.
BVF has made a $1-a-share tender offer for Avigen and is trying to replace the
directors. If it gains control, it could liquidate Avigen or sell it to
MediciNova, which has said it wants to buy it. Mr. Chahine, the chief of Avigen,
which is based in Alameda, Calif., said its assets might be parlayed into a deal
that would be worth more than BVF or MediciNova would pay and more than the
liquidation value. “All we’re saying is, give us an opportunity to canvass the
field, see what’s out there and bring something to the shareholders,” he said.
But Mr. Nodelman said such a process might eat up the company’s remaining cash.
“Someone’s got to police the space,” he said. “We’re making sure that the last
$50 million in the company don’t go to the bankers and the consultants and the
golden parachutes.”
BVF, which specializes in smaller biotech companies, has become the most
outspoken investor pressing for its money back. The fund, based in San
Francisco, gets about half of its capital from the Ziff family, which made its
fortune in magazine publishing.
Mr. Nodelman makes no apologies for BVF’s having bought Avigen stock again after
the collapse. The fund is also pressing for a cash-out to shareholders from
CombinatoRx. BVF has been a continuous shareholder in the company, although it
added to its stake after some CombinatoRx clinical trials failed.
CombinatoRx, whose strategy is to combine two old drugs to make one new one, has
lost $236 million since its inception in 2000. The company has about $1.45 a
share in cash, but its stock is trading for only 66 cents.
Alexis Borisy, the chief executive, said the company, based in Cambridge, Mass.,
was not ready for the grave. “We obviously think there’s a lot of upside value
in the CombinatoRx technology,” he said.
BVF and CombinatoRx are now in confidential discussions about the company’s
future.
BVF is also one of four investors, which collectively own about two-thirds of
the shares, demanding money back from Neurobiological Technologies of
Emeryville, Calif.
The company’s stroke drug is derived from the venom of the Malayan pit viper.
Three of the investors, including BVF, were shareholders when that drug failed
in a clinical trial in December. The fourth bought in after the failure. The
stock now trades at 58 cents, but its liquidation value would be as high as $1 a
share.
Matthew Loar, the chief financial officer, said the company was sympathetic to
the requests but had not yet decided what to do. In any case, he said, it could
not act as fast as the investors want.
“You can’t just turn off the lights in a company in a day,” he said. Among other
things, the company must figure out what to do with 1,000 poisonous snakes, he
said. “We’re going to get rid of them in the most expeditious, reasonable way
possible.”
Drug Investors Lose
Patience, NYT, 10.3.2009,
http://www.nytimes.com/2009/03/10/business/10biocash.html
Letters
An Ethics Debate at Harvard Med
March 9, 2009
The New York Times
To the Editor:
Re “Patching a Wound:
Working to End Conflicts at Harvard Medical” (Business Day, March 3):
Bravo to the Harvard medical students who are questioning medicine’s too-close
links to the pharmaceutical industry. It has been well demonstrated that the
outcome of research is often altered by the source of financing, even if the
researchers’ motives are pure.
The idealism of these young medical trainees should be heeded and acted upon,
especially as many of them may be the medical leaders of the future. A more
regulated and transparent approach would help restore integrity and trust to
modern medicine.
Steve Heilig
San Francisco, March 4, 2009
The writer is co-editor of The Cambridge Quarterly of Health Care Ethics.
•
To the Editor:
As a physician-scientist completing his training at a hospital affiliated with
Harvard Medical School, I can verify that the complex relationships between
medical faculty and the pharmaceutical industry can be fraught with conflicts of
interest. But I take issue with the implicit assumption that faculty members who
accept drug money are driven exclusively by greed; this issue is much more
complicated than it may seem at first.
Harvard Medical School’s “drug money” problem relates, in part, to a
little-known fact: Harvard rarely pays the salaries of its medical faculty
directly. Despite substantial revenue from tuition, most faculty members teach
medical students on a volunteer basis.
Similarly, the salaries of research faculty are not paid by Harvard, but rather
by grants from the National Institutes of Health, private foundations and in
some instances, grants from pharmaceutical companies. If these financing sources
dry up, professors are often forced to find a new job.
Thus, in many cases, money from the pharmaceutical industry doesn’t increase the
take-home pay of Harvard professors; it simply allows them to maintain their
employment.
Daniel Becker
Brookline, Mass., March 4, 2009
The writer is a fellow in the Renal Division of Brigham and Women’s Hospital and
a clinical fellow in medicine at Harvard Medical School.
•
To the Editor:
Your article implies that the “more than 200 Harvard Medical School students and
sympathetic faculty, intent on exposing and curtailing the industry influence in
their classrooms and laboratories,” are in complete agreement that industry and
private affiliation are detrimental to academic medical research. Rather, the
large number of students referred to signed a petition that advocated disclosure
of industry ties, not further severance of industry relations.
In fact, both “rival factions,” as the article described them, agree that
disclosure is important, but so are the relationships that foster quality
research, new medicines and appropriate compensation for expertise.
The implication that some Harvard physicians and researchers abuse their
academic posts for purely financial gains is unfounded. Many work tirelessly to
advance science and spend years with little compensation developing marketable
expertise in the clinic and the laboratory.
For example, in a course-based clinic centered on multiple myeloma treatment at
the Dana Farber Cancer Institute, we have seen firsthand how dramatic
improvements have been achieved through productive partnerships between academia
and industry.
Many of these researchers and physicians have given up potentially lucrative
careers in private practice or applied science to work in academia because of
their passion to educate future clinicians. Most work tirelessly to improve
patient care, advance their fields and collaborate on new treatment
possibilities.
As future physicians, we feel that disclosure of financial conflicts of interest
is essential to protecting our patients and that accurate information is
axiomatic to any medical curriculum. Our faculty, administration and students
operate within an institution that we believe provides superb patient care
through innovation, research and integrity.
Charles William Carspecken
Taylor Lloyd, Melina Marmarelis
Brian Thomas Kalish
Ashley R. Kochanek
Tomasz Stryjewski
Boston, March 4, 2009
The writers are members of the class of 2012 at Harvard Medical School.
•
To the Editor:
As a 1959 graduate of Harvard Medical School, looking forward three months to
the 50th reunion of my class, I am embarrassed to learn that my alma mater has
received an F grade from the American Medical Student Association for its poor
monitoring and controlling of drug industry money.
It is shocking that both the former dean and at least one current professor at
Harvard Medical School have served concurrently as highly paid board members of
pharmaceutical and medical products companies, and that, until recently,
professors have been allowed to serve, without disclosure in many cases, as
consultants to the manufacturers of the very products about which they teach.
Their students, many of whom will become the next generation of leaders in
American medicine, are being critically deprived of objective teaching. I am
afraid that their future patients will suffer.
The only good news in the article is that a start has been made at reform. I
hope that my reunion class will nudge this commendable effort along.
Cavin P. Leeman
New York, March 3, 2009
The writer is an emeritus clinical professor of psychiatry at SUNY Downstate
Medical Center.
An Ethics Debate at
Harvard Med, NYT, 9.3.2009,
http://www.nytimes.com/2009/03/09/opinion/l09harvard.html
Patient Money
Hanging On to Health Coverage,
if the Job Goes Away
March 7, 2009
The New York Times
By WALECIA KONRAD
If you’re fortunate to still have your job, but aren’t sure how much longer
that will be the case, lost income may not be your only worry. Your medical
insurance is at risk, too.
“When you’re still on the job, even if it’s just for a little while longer,
you’re in a slightly better position to make the most of the benefits you have
now and to figure out your options,” said the Oklahoma insurance commissioner,
Kim Holland, a longtime promoter of affordable health insurance.
She and other experts offer the following advice about girding for the worst
case.
Use it before you lose it. “My clients wouldn’t want to hear me say this,” said
Tom Billet, a senior executive with the corporate benefits consulting firm
Watson Wyatt. “But if you feel a layoff is pending, now is the time for you and
your family to get physicals, dental check-ups, eye exams and prescriptions
filled.”
That’s what Denise Young Farrell is doing. Ms. Farrell, the mother of two
children in Park Slope, Brooklyn, lost her job early this year when her
department at the Lifetime Networks cable channel moved to California. Her
husband lost his job at Bear Stearns last year. Ms. Farrell’s severance package
included two months of paid health care.
Check your benefits handbook to see how long your health care coverage will last
if you do lose your job. Often, employers will continue coverage until the last
day of the month in which the employee worked. So if your last day at work was
March 5, for example, you may have coverage until March 31, giving you a few
extra days for those doctor visits.
Sign onto your spouse’s plan. If your spouse has employer-sponsored family
health insurance benefits, he or she can add you and your dependents anytime
during the year. But do be aware of the deadlines. Most companies require any
changes to be filed within 30 or 60 days of the “qualifying event.” Depending on
your spouse’s company, that could mean the day you were laid off or your last
day of coverage.
In addition, some companies require written proof from your former employer that
you were laid off. To avoid snags, try to arrange this before your last day of
work. And be sure to check when the new coverage takes effect. If your spouse’s
plan has a three-month waiting period, for example, you’ll need to find
temporary coverage elsewhere.
Get to know Cobra. If you have health benefits in your current job, odds are
you’ll be eligible to continue purchasing that coverage temporarily under the
1986 law known by its acronym, Cobra.
Cobra requires employers with 20 or more workers to make health insurance
available to a former employee for up to 18 months after leaving the job —
regardless of whether you quit or were laid off. But because the former worker
must pay the full cost of that insurance, the premiums can easily exceed $1,000
a month for family coverage.
The new federal stimulus plan that President Obama recently signed into law does
provide some temporary relief for laid-off workers. But even if you qualify for
the subsidy, you’ll still pay 35 percent of the total health premium, compared
to the 10 or 15 percent you paid as an employee. So you might be paying $300 to
$400 or more a month. And that is for only the first 9 months of the 18-month
Cobra coverage. For the second nine months you’ll be paying full fare.
For fuller details on the new Cobra provisions, see this Congressional Web
page.If you do choose Cobra, pace yourself. Time it right, and you can
essentially get two months of free Cobra coverage.
After your last day of coverage under your employer’s plan, you have 63 days to
sign up to extend that coverage under Cobra. If you think you’re on the verge of
getting a new job, or if you’re trying to find a more affordable insurance
option, you can put off paying two months of Cobra premiums until you approach
the deadline. If the new job or alternate insurance works out, you will have
avoided those hundreds of dollars in Cobra premiums. But if you do fall ill or
get in an accident in the interim, you will be covered — as long as you pay
those back premiums.
Do be vigilant, though, about that 63-day deadline. Miss it, and you lose your
Cobra eligibility.
Try to negotiate health care as part of your severance. If you are eligible for
any type of severance, consider asking for an extension of health insurance in
exchange for a smaller cash payout. That will give you more time to research
your health insurance options and help you avoid a gap in coverage.
There is one caveat, said Kathryn Bakich, national health care compliance
director for the Segal Company, a benefits consulting firm: Avoid having your
company pay part or all of your Cobra premiums as part of a severance agreement.
Employers are still waiting for guidance on this point from the Department of
Labor and other government agencies, but if your former employer pays your Cobra
premiums directly, you may be ineligible for the new Cobra subsidy, according to
Ms. Bakich and other benefits experts. You’d be better off trying get a lump sum
payment that you could use to pay Cobra premiums, if extending your current
coverage isn’t an option.
When Cobra is not an option ... If you work for a small company (fewer than 20
employees) that doesn’t offer Cobra, 40 states offer what’s called mini-Cobra
continuation coverage that allows you to stay in your group plan. Some states
may offer the new Cobra subsidy in these plans. (Check with your state’s
insurance department.) If you do not have access to Cobra or a state
continuation plan, or if those benefits are close to running out, it’s important
to find insurance of some kind, whether it is group or individual.
Federal law mandates that at least one nongroup insurer in your state must
provide coverage to everyone, regardless of health issues. In many cases this is
your state’s high-risk insurance pool, but there are no limits on how much
insurers can charge for this coverage, so premiums can be extremely expensive.
For more information on what your state offers, go to the National Association
of Insurance Commissioners’ Web site, naic.org, to link to your state’s
insurance department.
If you have a flexible spending account — use it. Here’s a little known bonus in
the employee’s favor:
Let’s say you signed up to contribute $1,000 this year through payroll
deductions to your health care flexible spending account. So far you’ve only put
in about $200. No matter. “Companies must still reimburse you for the full
amount you’ve elected even if you haven’t contributed the total to the account
yet,” Mr. Billet said.
In this example, if you file claims for $1,000 of eligible health care expenses
before your last day on the job, you will get the full reimbursement — not just
the $200 you’ve paid in.
Hanging On to Health
Coverage, if the Job Goes Away, NYT, 7.3.2009,
http://www.nytimes.com/2009/03/07/health/policy/07patient.html?hp

Graham Roumieu
The Computer Will See You Now
NYT
6.3.2009
http://www.nytimes.com/2009/03/06/opinion/06coben.html?ref=opinion
Op-Ed Contributor
The Computer Will
See You Now
March 6, 2009
The New York Times
By ANNE ARMSTRONG-COBEN
FOR 20 years, I practiced pediatric medicine with a “paper
chart.” I would sit with my young patients and their families, chart in my lap,
making eye contact and listening to their stories. I could take patients’
histories in the order they wanted to tell them or as I wanted to ask. I could
draw pictures of birthmarks, rashes or injuries. I loved how patients could
participate in their own charts — illustrating their cognitive development as
they went from showing me how they could draw a line at age 2 and a circle at 3
to proudly writing their names at 5.
Now that I’ve been using a computer to keep patient records — a practice that I
once looked forward to — my participation with patients too often consists of
keeping them away from the keyboard while I’m working, for fear they’ll push a
button that implodes all that I have just documented.
We have all heard about the wonderful ways in which electronic medical records
are supposed to transform our broken health care system — by eradicating
illegible handwriting and enabling doctors to share patients’ records with one
another more easily. The recently passed federal stimulus package provides
doctors and hospitals with $17 billion worth of incentive payments to switch to
electronic records. The benefits may be real, but we should not sacrifice too
much for them.
The problem is not just with pediatrics. Doctors in every specialty struggle
daily to figure out a way to keep the computer from interfering with what should
be going on in the exam room — making that crucial connection between doctor and
patient. I find myself apologizing often, as I stare at a series of questions
and boxes to be clicked on the screen and try to adapt them to the patient
sitting before me. I am forced to bring up questions in the order they appear,
to ask the parents of a laughing 2-year-old if she is “in pain,” and to restrain
my potty mouth when the computer malfunctions or the screen locks up. I advise
teenagers to limit computer time as I sit before one myself for hours each day
until my own eyes twitch and my neck starts to spasm.
In short, the computer depersonalizes medicine. It ignores nuances that we do
not measure but clearly influence care. In the past, I could pick up a chart and
flip through it easily. Looking at a note, I could picture the visit and recall
the story. Now a chart is a generic outline, screens filled with clicked boxes.
Room is provided for text, but in the computer’s font, important points often
get lost. I have half-joked with residents that they could type “child has no
head” in the middle of a computer record — and it might be missed.
A box clicked unintentionally is as detrimental as an order written illegibly —
maybe worse because it looks official. It takes more effort and thought to write
a prescription than to pull up a menu of medications and click a box. I have
seen how choosing the wrong box can lead to the wrong drug being prescribed.
So before we embrace the inevitable, there should be more discussion and study
of electronic records, or at a minimum acknowledgment of the downside. A hybrid
may be the answer — perhaps electronic records should be kept only on tablet
computers, allowing the provider to write or draw, and to face the patient.
The personal relationships we build in primary care must remain a priority,
because they are integral to improved health outcomes. Let us not forget this as
we put keyboards and screens within the intimate walls of our medical homes.
Anne Armstrong-Coben is an assistant clinical professor of pediatrics at
Columbia.
The Computer Will See
You Now, NYT, 6.3.2009,
http://www.nytimes.com/2009/03/06/opinion/06coben.html?ref=opinion
Obama Tries to Start
Conversation on Health Care
March 5, 2009
By THE ASSOCIATED PRESS
The New York Times
Filed at 3:04 a.m. ET
WASHINGTON (AP) -- President Barack Obama has invited to the White House more
than 120 people who hold a wide range of views on how to fix the world's
costliest health care system, one that still leaves millions uninsured.
A broad group of doctors, patients, business owners and insurers were to gather
for a forum Thursday in hopes of building support for big changes in health
care. Republicans are invited, and they're expected to speak up.
''The president wants to engage with Congress in a transparent and bipartisan
fashion,'' said Melody Barnes, who heads White House domestic policy.
Among the invitees are some who helped kill the Clinton administration's health
care overhaul in the 1990s. Everyone is supposed to be on his best behavior, but
will that last?
''This is a different day, '' said Chip Kahn, a hospital lobbyist who opposed
President Bill Clinton's plan and was to attend Thursday's gathering. ''I think
among most of the stakeholders, everyone wants to see this work. There is a
tremendous feeling that it's time.''
Now president of the Federation of American Hospitals, Kahn worked for the
insurance industry in the Clinton years.
The difference this time, Obama argues, is that health care costs have become
unsustainable, particularly in a sinking economy. The U.S. spends $2.4 trillion
a year on health care, yet an estimated 48 million Americans lack coverage.
Obama's goal is health coverage for everyone.
Barnes said Obama is determined to pass health care legislation this year, and
while he wants it to be bipartisan, he will not be deterred by obstruction from
interest groups or ideological partisans.
''The president will make clear this has to be a bipartisan effort,'' Barnes
said. ''As for people who are there to set up hurdles, from his perspective that
isn't tolerable. It's crucial to families, businesses and our nation's budget
that we address the issue of exploding costs.''
Senate Republican leader Mitch McConnell of Kentucky released a letter to Obama,
saying his party is ready to work with the administration on health care, but
warning that reforms should not lead to a government-run system, and must
balance coverage expansions with curbs on costs.
In support of Obama's efforts, liberal activists have mobilized to keep the
pressure on Congress to pass legislation this year.
''It would be a mistake to dismiss this as a gabfest,'' Drew Altman, president
of the Kaiser Family Foundation, said about Obama's meeting. ''It's an effort to
keep the momentum going. The details are not going to be worked in two or three
hours at a White House summit.''
There were concerns Wednesday about some of those details.
Senate Finance Committee Chairman Max Baucus, D-Mont., who will play a leading
role in writing health care legislation, raised questions about the proposed
$634 billion ''down payment'' for expanded coverage that Obama included in the
2010 budget he released last week.
------
On the Net:
White House:
http://www.whitehouse.gov/agenda/health--care/
Obama Tries to Start
Conversation on Health Care, NYT, 5.3.2009,
http://www.nytimes.com/aponline/2009/03/05/washington/AP-Health-Care-Overhaul.html
Drug Approval
Is Not a Shield From Lawsuits,
Justices Rule
March 5, 2009
The New York Times
By DAVID STOUT
WASHINGTON — In one of the most important business cases in years, the
Supreme Court on Wednesday ruled that a drug company is not protected from
injury claims in state court merely because the federal government had approved
the products and its labeling.
The 6-to-3 ruling went in favor of a Vermont woman, a musician, who was awarded
more than $6 million after losing much of her arm following a botched injection
of an anti-nausea drug. It was a defeat for the Wyeth pharmaceutical company,
which had asked the justices to throw out the award, and by extension other
companies that might have pursued Wyeth’s line of argument in similar cases.
Ms. Levine had settled a parallel claim against the clinic where she was
treated.
The key issue before the justices was whether the Food and Drug Administration’s
approval of drug labels should control lawsuits in state courts contending, as
Ms. Levine’s did, that the labels did not contain adequate warnings. Wyeth’s
lawyers had argued that the company provided “ample, lavish warnings,” as one
attorney put it, and that Wyeth should not been held liable, because the Food
and Drug Administration had approved the label on the drug in question,
Phenergan.
But the high court held, in an opinion by Justice John Paul Stevens, that
Wyeth’s reading of the pertinent federal regulation was “cramped” and based on a
“fundamental misunderstanding.”
“It is a central premise of the Food, Drug and Cosmetic Act and the F.D.A.’s
regulations that the manufacturer bears responsibility for the content of its
label at all times,” the majority concluded in Wyeth v. Levine, No. 06-1249.
The majority upheld the Vermont Supreme Court, which in 2006 rejected Wyeth’s
argument that it had been put in an untenable position: having to comply with
federal law, given its requirement that the F.D.A. approve drug labels, and yet
being punished by the state jury’s verdict for not using a different, more
inclusive label.
Federal law “provides a floor, not a ceiling, for state regulation,” the Vermont
Supreme Court declared in the ruling that the United States Supreme Court
affirmed on Wednesday.
Ms. Levine’s suffering began in the spring of 2000 when, suffering from a
migraine, she visited a local clinic for a treatment she had received many
times: Demerol for pain and Phenergan for nausea.
If Phenergan is exposed to arterial blood, it causes swift and irreversible
gangrene. Therefore, it is typically administered by intramuscular injection.
Ms. Levine’s lawyers said an intravenous drip is also quite safe.
But a physician used a third method, injecting the drug into what she thought
was a vein, using a technique known as “IV push.” The assistant apparently
missed a vein and hit an artery instead, causing Ms. Levine’s right hand and
forearm to turn purple and black in the following weeks, leading to amputation
of much of her arm.
The F.D.A.-approved label warned that “inadvertent intra-arterial injection” can
cause gangrene requiring amputation, but it did not rule out administering the
drug by the “IV push” method.
The justices who sided with Ms. Levine on Wednesday said that “Wyeth could have
unilaterally added a stronger warning about IV-push administration” without
running afoul of federal regulations. Justices Anthony M. Kennedy, David H.
Souter, Ruth Bader Ginsburg and Stephen G. Breyer joined Justice Stevens, while
Justice Clarence Thomas filed an opinion concurring in the overall judgment.
Justice Samuel A. Alito Jr. wrote a dissent declaring, “This case illustrates
that tragic facts make bad law.” Joining him with Chief Justice John G. Roberts
Jr. and Justice Antonin Scalia.
Bert Rein, an attorney for Wyeth, said the company “fully complied with federal
law” in its labeling, and that the F.D.A. “is in the best position to weight the
risks and benefits of a medicine.”
Ms. Levine, now 63, was overjoyed. “Oh, my God. I’m so, so happy,” she told The
Associated Press in a telephone interview. “I’m just ecstatic. I’m going to have
to sit down.”
Drug Approval Is Not a
Shield From Lawsuits, Justices Rule, NYT, 5.3.2009,
http://www.nytimes.com/2009/03/05/washington/05scotus.html
Harvard Medical School
in Ethics Quandary
March 3, 2009
The New York Times
By DUFF WILSON
BOSTON — In a first-year pharmacology class at Harvard Medical School, Matt
Zerden grew wary as the professor promoted the benefits of cholesterol drugs and
seemed to belittle a student who asked about side effects.
Mr. Zerden later discovered something by searching online that he began sharing
with his classmates. The professor was not only a full-time member of the
Harvard Medical faculty, but a paid consultant to 10 drug companies, including
five makers of cholesterol treatments.
“I felt really violated,” Mr. Zerden, now a fourth-year student, recently
recalled. “Here we have 160 open minds trying to learn the basics in a protected
space, and the information he was giving wasn’t as pure as I think it should
be.”
Mr. Zerden’s minor stir four years ago has lately grown into a full-blown
movement by more than 200 Harvard Medical School students and sympathetic
faculty, intent on exposing and curtailing the industry influence in their
classrooms and laboratories, as well as in Harvard’s 17 affiliated teaching
hospitals and institutes.
They say they are concerned that the same money that helped build the school’s
world-class status may in fact be hurting its reputation and affecting its
teaching.
The students argue, for example, that Harvard should be embarrassed by the F
grade it recently received from the American Medical Student Association, a
national group that rates how well medical schools monitor and control drug
industry money.
Harvard Medical School’s peers received much higher grades, ranging from the A
for the University of Pennsylvania, to B’s received by Stanford, Columbia and
New York University, to the C for Yale.
Harvard has fallen behind, some faculty and administrators say, because its
teaching hospitals are not owned by the university, complicating reform; because
the dean is fairly new and his predecessor was such an industry booster that he
served on a pharmaceutical company board; and because a crackdown, simply put,
could cost it money or faculty.
Further, the potential embarrassments — a Senate investigation of several
medical professors, the F grade, a new state law effective July 1 requiring
Massachusetts doctors to disclose corporate gifts over $50 — are only now adding
to pressure for change.
The dean, Dr. Jeffrey S. Flier, who says he wants Harvard to catch up with the
best practices at other leading medical schools, recently announced a 19-member
committee to re-examine his school’s conflict-of-interest policies. The group,
which includes three students, is to meet in private on Thursday.
Advising the group will be Dr. David Korn, a former dean of the Stanford Medical
School who started work at Harvard about four months ago as vice provost for
research. Last year he helped the Association of American Medical Colleges draft
a model conflict-of-interest policy for medical schools.
The Harvard students have already secured a requirement that all professors and
lecturers disclose their industry ties in class — a blanket policy that has been
adopted by no other leading medical school. (One Harvard professor’s disclosure
in class listed 47 company affiliations.)
“Harvard needs to live up to its name,” said Kirsten Austad, 24, a first-year
Harvard Medical student who is one of the movement’s leaders. “We are really
being indoctrinated into a field of medicine that is becoming more and more
commercialized.”
David Tian, 24, a first-year Harvard Medical student, said: “Before coming here,
I had no idea how much influence companies had on medical education. And it’s
something that’s purposely meant to be under the table, providing information
under the guise of education when that information is also presented for
marketing purposes.”
The students say they worry that pharmaceutical industry scandals in recent
years — including some criminal convictions, billions of dollars in fines, proof
of bias in research and publishing and false marketing claims — have cast a bad
light on the medical profession. And they criticize Harvard as being less
vigilant than other leading medical schools in monitoring potential financial
conflicts by faculty members.
Dr. Flier says that the Harvard Medical faculty may lead the nation in receiving
money from industry, as well as government and charities, and he does not want
to tighten the spigot. “One entirely appropriate source, if done properly, is
industrial funds,” Dr. Flier said in an interview.
And school officials see corporate support for their faculty as all the more
crucial, as the university endowment has lost 22 percent of its value since last
July and the recession has caused philanthropic contributors to retrench. The
school said it was unable to provide annual measures of the money flow to its
faculty, beyond the $8.6 million that pharmaceutical companies contributed last
year for basic science research and the $3 million for continuing education
classes on campus. Most of the money goes to professors at the
Harvard-affiliated teaching hospitals, and the dean’s office does not keep track
of the total.
But no one disputes that many individual Harvard Medical faculty members receive
tens or even hundreds of thousands of dollars a year through industry consulting
and speaking fees. Under the school’s disclosure rules, about 1,600 of 8,900
professors and lecturers have reported to the dean that they or a family member
had a financial interest in a business related to their teaching, research or
clinical care. The reports show 149 with financial ties to Pfizer and 130 with
Merck.
The rules, though, do not require them to report specific amounts received for
speaking or consulting, other than broad indications like “more than $30,000.”
Some faculty who conduct research have limits of $30,000 in stock and $20,000 a
year in fees. But there are no limits on companies’ making outright gifts to
faculty — free meals, tickets, trips or the like.
Other blandishments include industry-endowed chairs like the three Harvard
created with $8 million from sleep research companies; faculty prizes like the
$50,000 award named after Bristol-Myers Squibb, and sponsorships like Pfizer’s
$1 million annual subsidy for 20 new M.D.’s in a two-year program to learn
clinical investigation and pursue Harvard Master of Medical Science degrees,
including classes taught by Pfizer scientists.
Dr. Flier, who became dean 17 months ago, previously received a $500,000
research grant from Bristol-Myers Squibb. He also consulted for three Cambridge
biotechnology companies, but says that those relationships have ended and that
he has accepted no new industry affiliations.
That is in contrast to his predecessor as dean, Dr. Joseph B. Martin. Harvard’s
rules allowed Dr. Martin to sit on the board of the medical products company
Baxter International for 5 of the 10 years he led the medical school,
supplementing his university salary with up to $197,000 a year from Baxter,
according to company filings.
Dr. Martin is still on the medical faculty and is founder and co-chairman of the
Harvard NeuroDiscovery Center, which researches degenerative diseases, and
actively solicits industry money to do so. Dr. Martin declined any comment.
A smaller rival faction among Harvard’s 750 medical students has circulated a
petition signed by about 100 people that calls for “continued interaction
between medicine and industry at Harvard Medical School.”
A leader of the group, Vijay Yanamadala, 22, said, “To say that because these
industry sources are inherently biased, physicians should never listen to them,
is wrong.”
Encouraging them is Dr. Thomas P. Stossel, a Harvard Medical professor who has
served on advisory boards for Merck, Biogen Idec and Dyax, and has written
widely on academic-industry ties. “I think if you look at it with intellectual
honesty, you see industry interaction has produced far more good than harm,” Dr.
Stossel said. “Harvard absolutely could get more from industry but I think
they’re very skittish. There’s a huge opportunity we ought to mine.”
Brian Fuchs, 26, a second-year student from Queens, credited drug companies with
great medical discoveries. “It’s not a problem,” he said, pointing out a
classroom window to a 12-story building nearby. “In fact, Merck is right there.”
Merck built a corporate research center in 2004 across the street from Harvard’s
own big new medical research and class building. And Merck underwrites plenty of
work on the Harvard campus, including the immunology lab run by Dr. Laurie H.
Glimcher — a professor who also sits on the board of the drug maker
Bristol-Myers Squibb, which paid her nearly $270,000 in 2007.
Dr. Glimcher says industry money is not only appropriate but necessary. “Without
the support of the private sector, we would not have been able to develop what I
call our ‘bone team’ in our lab,” she said at a recent student and faculty forum
to discuss industry relationships. Merck is counting on her team to help come up
with a successor to Fosamax, the formerly $3 billion-a-year bone drug that went
generic last year. But Dr. Marcia Angell, a faculty member and former editor in
chief of The New England Journal of Medicine, is among the professors who argue
that industry profit motives do not correspond to the scientific aims of
academic medicine and that much of the financing needs to be not only disclosed,
but banned. Too many medical schools, she says, have struck a “Faustian bargain”
with pharmaceutical companies.
“If a school like Harvard can’t behave itself,” Dr. Angell said, “who can?”
Harvard Medical School
in Ethics Quandary, NYT, 3.3.2009,
http://www.nytimes.com/2009/03/03/business/03medschool.html?ref=opinion
Abuse Is Found
at Psychiatric Unit
Run by the City
February 6, 2009
The New York Times
By ANEMONA HARTOCOLLIS
The federal government has documented a pattern of sexual and other violent
assaults among patients at the psychiatric unit of a city-run Brooklyn hospital
where a woman died in June on the floor of the emergency waiting room while
staff members ignored her.
After a yearlong investigation, the Department of Justice portrayed the unit at
Kings County Hospital Center as a nightmarish place where patients were not
treated for suicidal behavior, were routinely subdued with physical restraints
and drugs instead of receiving individualized psychiatric treatment, and were
frequently abused by other patients.
The details are laid out in a 58-page report to Mayor Michael R. Bloomberg that
was made public on Thursday.
The investigators found that the psychiatric service operated like a prison. The
report said that instead of meaningful treatment and diagnosis, the patients
received frequent visual checks by the staff, and that even when patients were
supposedly under watch, violence and attempted suicides occurred.
Among the most serious incidents the report documented were an October brawl
among six patients that left one needing surgery, and an autistic patient being
forced to perform oral sex in November. The report also included allegations
that a woman was raped and that a 14-year-old was forced to engage in oral sex
by a 16-year-old.
All four incidents occurred after the highly publicized death of Esmin Green, a
Jamaican immigrant with a history of depression, who collapsed on the floor of
the emergency waiting room after waiting nearly 24 hours to be seen. A
surveillance video showed Ms. Green, 49, lying on the floor for nearly an hour;
during that time, a guard came in to check on her by wheeling his chair along,
and another staff member prodded her with a foot.
“While perhaps unique in the extent of the harm that resulted, the tragic case
of Ms. Green typifies the patterns of inadequate care and treatment,” reads the
report, from Loretta King, an acting assistant attorney general, and Benton J.
Campbell, the United States attorney in Brooklyn.
The report, a summary account of the federal investigation that resulted from a
2007 lawsuit by the New York Civil Liberties Union and others, found at least
three cases, including Ms. Green’s, when employees falsified records to hide
their neglect.
The report became public when Alan D. Aviles, president of the city’s Health and
Hospitals Corporation, convened a news conference on Thursday to announce that
“radical changes” had been made at Kings County, which treats many of the city’s
most severely mentally ill. While questioning some details of the report, he
admitted that the unit “too often failed” its patients.
At the hospital’s new $153 million building in central Brooklyn, he announced
the replacement of its top two administrators and the addition of 200 medical
personnel to its 600-member staff.
Mr. Aviles also outlined new protocols for screening emergency-room admissions,
using nonuniformed security officers trained in crisis intervention rather than
hospital police. Mr. Aviles noted that in Ms. Green’s case, two guards had
looked in on her but decided that she was not their responsibility.
“They clearly felt disconnected from the treatment team,” Mr. Aviles said. “This
says something very damning about the model.”
Mr. Aviles said the hospital had cut the average time in the emergency
department to 8 hours from 27, and that the number of patients waiting seldom
exceeded 25 now, compared with 50 or more on occasion.
“It would be disingenuous of me to suggest that we could prevent all such future
incidents, but we can do better,” he said.
Stu Loeser, a City Hall spokesman, said that the mayor believed that the Justice
Department report raised “serious issues” but that the changes Mr. Aviles
announced “go a long way to addressing many of the conditions.”
The Justice Department’s report said conditions at the psychiatric unit were
“highly dangerous and require immediate attention.” It added: “Substantial harm
occurs regularly due to K.C.H.C.’s failure to properly assess, diagnose,
supervise, monitor and treat its mental health patients.”
The report said that many patients were admitted with “catch-all” diagnoses and
that the staff used “boilerplate forms and checklists” rather than writing
“individualized narratives.”
The report said that patients were often left in restraints for the two-hour
limit even though they had changed their behavior, suggesting that the
confinement was punishment rather than therapy. And investigators found it was
common to administer injections of more than one antipsychotic medication
simultaneously, despite the risk of side effects and overdosing.
In one case, a patient’s treatment plan did not address his obesity, high blood
pressure and diabetes, until he had a stroke, according to the report.
Abuse Is Found at
Psychiatric Unit Run by the City, NYT, 6.2.2009,
http://www.nytimes.com/2009/02/06/nyregion/06kings.html?hp
Editorial
Your E-Health Records
February 1, 2009
The New York Times
As part of the stimulus package, $20 billion will be pumped into the health
care system to accelerate the use of electronic health records. The goal is both
to improve the quality and lower the costs of care by replacing cumbersome paper
records with electronic records that can be easily stored and swiftly
transmitted.
The idea is sound, but it also raises important questions about how to ensure
the privacy of patients. Fortunately, the legislation would impose sensible
privacy protections despite attempts by business lobbyists to weaken the
safeguards.
With paper records the opportunities for breaches are limited to
over-the-shoulder glimpses or the occasional lost or stolen files. But when
records are kept and transferred electronically, the potential for abuse can
become as vast as the Internet.
Electronic health records that can be linked to individual patients are already
protected by laws that apply primarily to hospitals, doctors, nursing homes,
pharmacists, laboratories and insurance plans. The stimulus bill that has passed
in the House, and a similar bill awaiting approval in the Senate, would
strengthen the privacy requirements and apply them more directly to “business
associates” of the providers, like billing and collection services or pharmacy
benefit managers, that have access to sensitive data but are not readily held
accountable for any misuse.
The potential for harm was spelled out by the American Civil Liberties Union in
a recent letter to Congress. Employers who obtain medical records
inappropriately might reject a job candidate who looks expensive to insure. Drug
companies with access to pharmaceutical records might try to pressure patients
to switch to their products. Data brokers might buy medical and pharmaceutical
records and sell them to marketers. Unscrupulous employees with access to
electronic records might snoop on the health of their colleagues or neighbors.
The bills pending in Congress would go a long way toward preventing such abuses.
They would outlaw the sale of any personal health information without the
patient’s permission, mandate audit trails to help detect inappropriate access,
and require that patients be notified whenever their records are lost or used
for an unauthorized purpose. They would also beef up the penalties for
noncompliance and allow state attorneys general to help enforce the rules — a
useful backup in case the federal government falls down on the job. The House
version would also encourage the use of protective technologies, like
encryption, to protect personal medical information that will be transmitted.
Health insurance plans and some disease management groups are complaining that
the new requirements would impose administrative burdens that could actually
impede the use of electronic records and interfere with coordination of care.
They want to ease the marketing restrictions, notify patients only if security
breaches are harmful, and keep the attorneys general out of the enforcement
role.
It should be possible through implementing regulations to fine-tune the privacy
requirements so that they do not disrupt patient care. Congress must make every
effort to ensure that patients’ privacy is protected.
Your E-Health Records,
NYT, 1.2.2009,
http://www.nytimes.com/2009/02/01/opinion/01sun2.html?ref=opinion
Pfizer Agrees to Pay $68 Billion
for Rival Drug Maker Wyeth
January 26, 2009
The New York Times
By ANDREW ROSS SORKIN
and DUFF WILSON
The board of Pfizer, the world’s largest drug maker, agreed to acquire a
rival, Wyeth, for $68 billion, the companies announced Monday.
The deal, if completed, would not only create a pharmaceutical behemoth but
would be a rarity in the current financial tumult: a big acquisition that is not
a desperate merger of two banks orchestrated by the government.
It will also be the first big merger backed by Wall Street in months. While
credit has been notoriously tight of late, five banks have agreed to lend Pfizer
$22.5 billion to pay for the deal. Pfizer, which has roughly $26 billion in
cash, would finance the remainder through a combination of cash and stock.
If completed as planned, the transaction would be the biggest merger since AT&T
and BellSouth combined in a $70 billion deal in March 2006, according to the
research firm Capital IQ.
Pfizer also said Monday that its net income for the fourth quarter dropped 90
percent from the period a year ago, citing a charge to resolve inquires into its
off-label promotional practices. In its statement, it also said that it planned
to its work force by about 10 percent and reduce the number of manufacturing
sites. The company also said that it would cut its dividend.
Pfizer earned $268 million, or 4 cents a share, in the fourth quarter, down from
$2.72 billion, or 40 cents a share, in the period a year ago. Revenue dropped 4
percent, to $12.35 billion. Excluding the $2.3 billion to settle the marketing
inquiry, Pfizer had a profit of 65 cents a share. Analysts surveyed by Thomson
Reuters had forecast 59 cents.
“The combination of Pfizer and Wyeth will meaningfully deliver Pfizer’s
strategic priorities in a single transaction,” the Pfizer’s chief executive,
Jeffrey B. Kindler, said in a statement Monday. “Our combined company will be
one of the most diversified in the industry and will benefit from complementary
patient-centric units that match speed with the benefits of a global company’s
scale and resources.”
The merger almost came unhinged at the 11th hour. While the boards of both
companies agreed to the broad outlines of the deal and its price before the
weekend, these people said, one issue was still a sticking point: whether Pfizer
would be allowed to back out of the deal if the economy worsened or Wyeth’s
prospects faded.
In better times, deals often falter on matters of strategy or price. But in this
case, because of the ailing economy, Pfizer has agreed to pay a staggering
breakup fee, $4.5 billion, if it does not complete the deal under certain
circumstances — if, for example, its credit rating drops and it can no longer
finance the deal. That is almost twice the typical breakup fee for a deal of
this size.
If the acquisition is completed, it may demonstrate that Wall Street is willing
to lend again, at least to the nation’s top companies with the best credit
ratings.
“If banks need to send a message that they’re loaning, they want to be loaning
to this quality of company,” said Catherine Arnold, an analyst at Credit Suisse.
Pfizer’s bid is being financed by four banks that received federal bailout
money: Goldman Sachs, JPMorgan Chase, Citigroup and Bank of America, the people
involved in the deal said. Such banks have been criticized for not doing more
lending since they received the government aid.
Barclays, which acquired Lehman Brothers out of bankruptcy in the fall, is also
providing financing, these people said.
Pfizer appears to be taking advantage of the bad market for credit to buy Wyeth
at a lower price than it might fetch if competing bids were to emerge, which
analysts do not expect.
“They have a unique opportunity now because not everybody can get that capital,”
said Barbara Ryan, an analyst at Deutsche Bank.
Because the combined company is expected to generate more than $20 billion in
cash a year, Ms. Ryan said, “even when they borrow money, they will still have
plenty of revenue.”
Under the terms of the deal, Pfizer would pay $50.19 a share for the company —
$33 a share in cash and 0.985 Pfizer shares worth $17.19 a share based on
Pfizer’s closing price on Friday. That is roughly a 29 percent premium over the
share price before word of the deal leaked on Friday.
Both companies’ boards of directors approved the deal.
Wyeth’s management team would depart, the people involved in the negotiations
said. Pfizer is also planning to cut its quarterly dividend in half to 16 cents,
these people said, in an effort to maintain its credit rating.
After news reports disclosed the talks on Friday, investors applauded the
possibility of a deal. Shares of Wyeth rose $4.91, or 12.6 percent, to close at
$43.74. Pfizer climbed 24 cents, or 1.4 percent, to close at $17.45.
Pfizer expects to save $4 billion annually by combining with Wyeth; those
savings will be phased in over three years.
Mr. Kindler of Pfizer, first approached Wyeth last spring with a phone call,
people involved in the talks said. The negotiations heated up in the summer but
appeared to collapse when the banking system went into a tailspin in September
and October.
Since then, there were several brief moments when it appeared the deal would
move ahead, but then the talks would fall apart once again, usually over
financing, these people said. It was apparently only within the last week or so
that the financing commitment came together.
For Mr. Kindler, a lawyer who came to Pfizer from McDonald’s, the deal may be a
job-saver. His and the company’s most pressing challenge has been the impending
expiration of patent rights to the cholesterol-lowering drug Lipitor — which
accounted for a quarter of the company’s 2007 revenue of $48 billion and remains
the best-selling drug in the world. The patent ends in 2011.
Still, even with the Wyeth deal, much would remain undone for Pfizer as it faces
product, patent and pipeline problems for other drugs as well.
“It’s not just Lipitor,” Ms. Arnold wrote last year in a report to investors.
Pfizer faces a run of 14 patent expirations through 2014, which would add up to
lost revenue of about $35 billion as those drugs give way to cheap generics,
according to Ms. Arnold. Pfizer’s patent problem is not unique among the big
drug makers. Merck, Bristol Myers Squibb and Eli Lilly are all facing their own
patent losses in the next five years. “Everybody’s staring at the same
challenges down the road,” Ms. Ryan said.
She said that Mr. Kindler, who became chief executive in July 2006, had probably
not been in a position to make a play for a company like Wyeth until after he
had cut costs, revamped Pfizer’s core business and accepted the reality that the
research pipeline was not producing blockbusters. “Hope springs eternal from the
research pipeline,” Ms. Ryan said.
As part of the deal with Wyeth, both companies will have to repatriate tens of
billions of dollars back into the United States, which could have a high tax
cost. Pfizer reported $25.3 billion in revenue, 52.2 percent of its total, from
overseas operations in 2007, according to securities filings.
If foreign profits were repatriated to the United States, Pfizer would have to
pay the difference between the tax paid in the foreign country, as low as 5
percent in Ireland, for example, and the 35 percent tax rate in the United
States.
Ms. Arnold said some tax penalties might be expected, but could be reduced by
doing some of the buying and selling overseas.
“The experts that we’ve spoken to have very definitely said you can use offshore
cash to buy offshore assets, and Pfizer and Wyeth both have very significant
offshore subsidiaries that they place cash in,” Ms. Arnold said. For example,
she said, “Pfizer Ireland can use its cash to buy Wyeth Ireland or Wyeth
Singapore.”
Wyeth, with sales of about $23 billion for the 12 months that ended Sept. 30,
has about $2.7 billion in cash and liquid assets, according to David S.
Moskowitz, an analyst at Caris & Company, an investment bank.
Pfizer was advised by Goldman, JPMorgan and Barclays; Wyeth was advised by
Morgan Stanley and Evercore Partners.
Erik Gordon, a professor at the Ross School of Business at the University of
Michigan who follows biomedical industries, said Pfizer and Wyeth were a great
fit that made the deal creditworthy.
First, because Pfizer has so much cash, the deal does not have to be highly
leveraged with debt, Mr. Gordon said. Second, the two companies have enough
overlap that they can achieve considerable saving through consolidating
duplicate operations and cutting costs. And finally, parts of a combined
operation could be spun off to raise money.
Mr. Gordon pointed to the animal health businesses of both companies — which,
considered together, accounted for $2.8 billion in revenue and about $600
million in profit in the first nine months of 2008.
“They could sell that business for billions of dollars to either pay down the
debt or service the debt,” he said. In addition, he said Pfizer could resell
Wyeth’s consumer products business. He added: “This deal is the rare thing.
This’ll be the only money investment bankers make in a while.”
Pfizer Agrees to Pay $68
Billion for Rival Drug Maker Wyeth, NYT, 26.1.2009,
http://www.nytimes.com/2009/01/26/business/26drug.html?hp
F.D.A. Approves a Stem Cell Trial
January 23, 2009
The New York Times
By ANDREW POLLACK
In a research milestone, the federal government will allow the world’s first
test in people of a therapy derived from human embryonic stem cells.
Federal drug regulators said that political considerations had no role in the
decision. Nevertheless, the move coincided with the inauguration of President
Obama, who has pledged to remove some of the financing restrictions placed on
the field by President George W. Bush.
The clearance of the clinical trial — of a treatment for spinal cord injury — is
to be announced Friday by Geron, the biotechnology company that first applied to
the Food and Drug Administration to conduct the trial last March. The F.D.A. had
first said no, asking for more data.
Thomas B. Okarma, Geron’s chief executive, said Thursday that he did not think
that the Bush administration’s objections to embryonic stem cell research played
a role in the F.D.A.’s delaying approval.
“We really have no evidence,” Dr. Okarma said, “that there was any political
overhang.”
But others said they suspected it was more than a coincidence that approval was
granted right after the new administration took office.
“I think this approval is directly tied to the change in administration,” said
Robert N. Klein, the chairman of California’s $3 billion stem cell research
program. He said he thought the Bush administration had pressured the F.D.A. to
delay the trial.
Mr. Klein called the approval of the first human trial of this sort “an
extraordinary benchmark.”
Stem cells derived from adults and fetuses are already being used in some
clinical trials, but they generally have less versatility than embryonic stem
cells in terms of what tissue types they can form.
The F.D.A. approval comes a little more than 10 years after the first human
embryonic stem cells were isolated at the University of Wisconsin, in work
financed by Geron.
Because the cells can turn into any type of cell in the body, the theory is they
may one day be able to provide tissues to replace worn-out organs or
nonfunctioning cells to treat diabetes, heart attacks and other diseases. The
field is known as regenerative medicine.
The Bush administration restricted federal financing for research on embryonic
stem cells because creation of the cells entails the destruction of human
embryos.
Geron’s trial will involve 8 to 10 people with severe spinal cord injuries. The
cells will be injected into the spinal cord at the injury site 7 to 14 days
after the injury occurs, because there is evidence the therapy will not work for
much older injuries.
The study is a so-called Phase I trial, aimed mainly at testing the safety of
the therapy. There would still be years of testing and many hurdles to overcome
before the treatment would become routinely available to patients.
Geron, which is based in Menlo Park, Calif., said that it had identified up to
seven medical centers for the trial but that those sites must first get
permission from their own internal review boards to participate.
Even as some researchers hailed the onset of clinical trials, others expressed
trepidation that if the therapy proves unsafe — or even if it is safe but does
not work — it could cause a backlash that would set the field back for years.
“It would be a disaster, a nightmare, if we ran into these kinds of problems in
this very first trial,” said Dr. John A. Kessler, the chairman of neurology and
director of the stem cell institute at Northwestern University.
Dr. Kessler, whose own daughter was paralyzed from the waist down in a skiing
accident, said he thought Geron’s therapy was not the ideal candidate for the
first trial. He said results showing the therapy worked in moderately injured
animals might not apply to more seriously injured people.
“We really want the best trial to be done for this first trial, and this might
not be it,” he said.
Dr. Okarma of Geron emphasized that the purpose of the first trial was safety,
so that lack of efficacy should not be a problem. While researchers will also
look for signs the treatment works, he said, the best that could be hoped for
would be some slight restoration of function that could then be enhanced through
physical therapy.
“We don’t expect to take someone who is completely paralyzed from the waist down
and have them dance six months later,” he said. If the first trial shows safety,
the company would then hope to test higher doses of cells and treat patients
with less severe injuries, he said.
Geron’s therapy involves using various growth factors to turn embryonic stem
cells into precursors of neural support cells called oligodendrocytes, which are
then injected into the spinal cord at the site of the injury.
The hope is that the injected cells will help repair the insulation, known as
myelin, around nerve cells, restoring the ability of some nerve cells to carry
signals. There is also some hope that growth factors produced by the injected
cells will spur damaged nerve cells to regenerate.
The therapy was developed in collaboration with Hans Keirstead of the University
of California, Irvine. He has shown videos of paralyzed rats that were able to
walk again, albeit imperfectly, after receiving the therapy. Those videos helped
persuade California voters to approve the $3 billion stem cell research program
in 2004.
The main safety concern is that if raw embryonic cells are put into the body,
they can form tumors. Even though most such tumors do not spread like other
cancers, any unwanted growth in the spinal cord can further damage nerves.
“It’s not ready for prime time, at least not in my mind, until we can be assured
that the transplanted stem cells have completely lost the capacity for
tumorogenicity,” said Dr. Steven Goldman, chairman of neurology at the
University of Rochester. He was a member a committee convened by the F.D.A. last
April to examine the safety aspects of trials using therapies from embryonic
stem cells.
Dr. Okarma said Geron had done numerous studies showing that its cells did not
contain residual embryonic cells and did not form tumors in animals even after a
year. It submitted 22,000 pages of data to the F.D.A., perhaps the largest
application ever for permission to begin a clinical trial.
The embryonic stem cell line used by Geron is one of the oldest ones and was
therefore eligible for federal financing under the Bush administration’s policy,
Dr. Okarma said.
Nevertheless, Geron paid for its own work, spending $45 million to prepare its
F.D.A. application.
Geron, which was formed in 1990 as an antiaging company, is still in the
development stage and is not yet profitable, having lost about $500 million
since its inception. Besides working on stem cells, it is testing drugs for
cancer that influence telomeres, the caps on the ends of chromosomes that help
control the aging of cells. Geron’s market value is about $400 million.
While the Bush administration’s policy did not impede the company’s application
at the F.D.A., Dr. Okarma said, it did slow progress for the field in general by
making it hard for academics to do research.
“It is the private sector that has kept the technology alive so that it can see
the light of day in a clinical trial,” he said.
Mr. Klein of the California stem cell program said he thought the next trial
might be of a treatment for macular degeneration, an eye disease, that is being
developed in Britain.
In the last couple of years, some attention has turned away from embryonic stem
cells to a newer technique that allows a patient’s own skin cells to be turned
into a cell resembling such embryonic cells.
That might do away with the need for embryos. And the resulting tissue made from
those cells would match the patient, doing away with the need for immune
suppression to prevent rejection of the transplant. Geron said its trial would
require only temporary use of low doses of immune-suppressing drugs.
But the newer technique involves putting genes into the skin cells using
viruses, which also raises a risk of cancer.
F.D.A. Approves a Stem
Cell Trial, NYT, 24.1.2009,
http://www.nytimes.com/2009/01/23/business/23stem.html?hp
Alexandra Falagara
The Abortion Choices of Poor Women
NYT
12.1.2009
http://www.nytimes.com/2009/01/12/opinion/l12abort.html
Letters
The Abortion
Choices of Poor Women
January 12, 2009
The New York Times
To the Editor:
Re “For Privacy’s
Sake, Risking Do-It-Yourself Abortion” (news article, Jan. 5):
Throughout history, women have tried many ways to end an unwanted pregnancy.
Your article underscores the fact that after 36 years of legal abortion, some
women still struggle to obtain reproductive health care because they don’t
understand or trust the American medical system.
In my Washington Heights clinic in northern Manhattan, I see at least one
patient every week who has tried to end a pregnancy on her own. When lack of
information, poverty or stigma overwhelm pregnant women, some take matters into
their own hands.
We can do better to educate women about their legal options. While they can use
misoprostol to end a pregnancy, there are safer and more effective methods.
As we approach the anniversary of Roe v. Wade, physicians need to remember that
reproductive health care remains a challenge, even in New York City. We must
work within our communities to ensure that women know how to navigate the health
care system.
Anne Davis
New York, Jan. 5, 2009
The writer is medical director of Physicians for Reproductive Choice and Health.
•
To the Editor:
In your article, abortion is called “safe, legal and inexpensive.”
While this common procedure is safe and is mostly legal, despite aggressive
regulation, it is not inexpensive. An abortion at 10 weeks’ gestation costs $523
on average, often out of pocket.
To term this “inexpensive,” especially in the current economy, is ludicrous.
Many women struggle to raise the money for an abortion as their pregnancy
progresses. Sixty-seven percent of poor women having an abortion report that
they would have preferred to have an earlier procedure.
Regarding abortion’s “widespread availability,” that is perhaps true for some in
New York, but not for all women.
Self-induced abortion does raise questions about women’s experience, but
glossing over the challenges of gaining access to abortion services does nothing
to answer these questions. It neither reflects the reality of abortion delivery
nor the reality of women’s lives.
Melanie Zurek
Silvia Henriquez
Cambridge, Mass., Jan. 6, 2009
The writers are executive directors of, respectively, the Abortion Access
Project and the National Latina Institute for Reproductive Health.
•
To the Editor:
Your article points out that for some women abortion care has not changed in
more than 100 years.
Using the code “I need to bring down my period,” women in Washington Heights ask
the pharmacist for an abortion drug.
In the 1800s, newspapers and the religious press in the United States carried
ads for medicines that would cure “blocked” menstruation. Such ads stated that
the product should not be used by married ladies. This was a code that the
product could cause an abortion, thus telling women who wanted to end a
pregnancy to buy the product.
On the other hand, this is the 21st century. Some teenagers are going to the
Internet for instructions on a do-it-yourself abortion. I went online to see
what information was out there and was horrified by what I found.
On one site the reader is instructed to insert implements vaginally and then
head for the emergency room once the hemorrhaging starts. But as a pediatric
nurse practitioner in Washington Heights, I know there are clinics that provide
safe, confidential care for teenagers.
Confidential care is the law of the state. One would hope that pharmacists would
tell women about these facilities. New York women do not have to live with
19th-century care. Poor women in other states may not have a choice.
Carol Roye
Pleasantville, N.Y., Jan. 5, 2009
The writer is assistant dean (acting) for research and a professor of nursing at
Hunter College.
•
To the Editor:
Your article is an excellent reminder of the struggles facing poor women who
seek basic reproductive health care in this country. Because of a law called the
Hyde Amendment, which was first enacted in 1976 and renewed each year since,
women on Medicaid are not covered for the cost of an abortion.
Unlike other basic health needs for men and women, this procedure, which nearly
one-third of all women in the United States will have by the end of their
reproductive years, is specifically carved out of coverage. For poor women in
Washington Heights, paying $30 for a risky and illegal drug is much more
realistic than paying several hundred dollars (or much more) for a legal
abortion out of their own pockets.
If President-elect Barack Obama is serious about improving the health of poor
women, one of his first agenda items should be the repeal of the Hyde Amendment.
In the meantime, poor women will, at best, turn to private assistance through
local abortion funds, or, at worst, turn to risky medicine that is the modern
equivalent of the back-alley abortion.
David S. Cohen
Philadelphia, Jan. 5, 2009
The writer is an associate professor at the Drexel University Earle Mack School
of Law and a board member of the Women’s Medical Fund in Philadelphia.
•
To the Editor:
The article about Dominican women in New York who use medication to give
themselves abortions highlights the need for better information.
In some states, legal residents and/or undocumented immigrants may be eligible
for medical programs that would pay for abortion or contraception. Where women
have no access to publicly funded medical care, they may still find assistance
from a local abortion fund or from the National Network of Abortion Funds, which
helps women who cannot afford an abortion to obtain safe pregnancy termination
services.
Access to information about these resources may not be enough to combat the fear
of deportation and social censure, but it is a start.
Rachel Roth
Arlington, Mass., Jan. 7, 2009
The writer is co-author of a report about abortion financing.
The Abortion Choices of
Poor Women, NYT, 12.1.2009,
http://www.nytimes.com/2009/01/12/opinion/l12abort.html
For
Privacy’s Sake,
Taking Risks to End Pregnancy
January 5, 2009
The New York Times
By JENNIFER 8. LEE
and CARA BUCKLEY
Amalia Dominguez was 18 and desperate and knew exactly what to
ask for at the small, family-run pharmacy in the heart of Washington Heights,
the thriving Dominican enclave in northern Manhattan. “I need to bring down my
period,” she recalled saying in Spanish, using a euphemism that the pharmacist
understood instantly.
It was 12 years ago, but the memory remains vivid: She was handed a packet of
pills. They were small and white, $30 for 12. Ms. Dominguez, two or three months
pregnant, went to a friend’s apartment and swallowed the pills one by one,
washing them down with malta, a molasseslike extract sold in nearly every bodega
in the neighborhood.
The cramps began several hours later, doubling Ms. Dominguez over, building and
building until, eight and a half hours later, she locked herself in the bathroom
and passed a lifeless fetus, which she flushed.
The pills were misoprostol, a prescription drug that is approved by the Food and
Drug Administration for reducing gastric ulcers and that researchers say is
commonly, though illegally, used within the Dominican community to induce
abortion. Two new studies by reproductive-health providers suggest that improper
use of such drugs is one of myriad methods, including questionable homemade
potions, frequently employed in attempts to end pregnancies by women from
fervently anti-abortion cultures despite the widespread availability of safe,
legal and inexpensive abortions in clinics and hospitals.
One study surveyed 1,200 women, mostly Latinas, in New York, Boston and San
Francisco and is expected to be released in the spring; the other, by Planned
Parenthood, involved a series of focus groups with 32 Dominican women in New
York and Santo Domingo. Together, they found reports of women mixing malted
beverages with aspirin, salt or nutmeg; throwing themselves down stairs or
having people punch them in the stomach; and drinking teas of avocado leaf, pine
wood, oak bark and mamon fruit peel.
Interviews with several community leaders and individual women in Washington
Heights echoed the findings, and revealed even more unconventional methods like
“juice de jeans,” a noxious brew made by boiling denim hems.
“Some women prefer to have a more private experience with their abortion, which
is certainly understandable,” said Dr. Daniel Grossman, an obstetrician with
Ibis Reproductive Health in San Francisco, which joined Gynuity Health Projects
in New York in conducting the larger study. “The things they mention are, ‘It is
easier.’ It was recommended to them by a friend or a family member.”
Dr. Carolyn Westhoff, an obstetrician at NewYork-Presbyterian/Columbia
University Medical Center, said the trend fits into a larger context of
Dominicans seeking home remedies rather than the care of doctors or hospitals,
partly because of a lack of insurance but mostly because of a lack of trust in
the health care system. “This is not just a culture of self-inducted abortion,”
she said. “This is a culture of going to the pharmacy and getting the medicine
you need.”
Physicians say that women can obtain the pills either through pharmacies that
are willing to bend the rules and provide the medicine without a prescription or
by having the drugs shipped from overseas.
It is impossible to know how many women in New York or nationwide try to end
their pregnancies themselves, but in the vibrant, socially conservative
Dominican neighborhoods of Upper Manhattan, the various methods are passed like
ancient cultural secrets. In a study of 610 women at three New York clinics in
largely Dominican neighborhoods conducted eight years ago, 5 percent said they
had taken misoprostol themselves, and 37 percent said they knew it was an
abortion-inducing drug. Doctors and community leaders say they have not seen any
signs of the phenomenon disappearing, which they find worrisome because of
concerns about the drug’s effectiveness and potential side effects.
Sold under the brand name Cytotec, misoprostol is approved to induce abortion
when taken with mifepristone, or RU-486; doctors also sometimes use it to induce
labor, though it is not approved for that use. A spokesman for Pfizer, which
manufacturers Cytotec, declined to comment beyond saying that the company does
not support the off-label use of its products and noting that the label includes
“F.D.A.’s strongest warning against use in women who are pregnant.”
That warning, in capital letters, also notes that the drug “can cause abortion.”
But it does not always do so, not least because notions of how best to use it
vary from inserting several pills into the vagina to letting them dissolve under
the tongue. The side effects can be serious, and include rupture of the uterus,
severe bleeding and shock.
“We do worry because we don’t know where women are getting the instructions
from,” said Jessica Gonzalez-Rojas of the National Latina Institute for
Reproductive Health, which was also a partner on the Ibis study. “We imagine
that there is misinformation on how to take it, which is why it could be hit or
miss.”
In 2007 in Massachusetts, an 18-year-old Dominican immigrant named Amber Abreu
took misoprostol in her 25th week of pregnancy and gave birth to a 1-pound baby
girl who died four days later; a judge sentenced her in June to probation and
ordered her into therapy. In South Carolina in February, a Mexican migrant farm
worker, Gabriela Flores, pleaded guilty to illegally performing an abortion and
was sentenced to 90 days in jail for taking misoprostol while four months
pregnant in 2004. A Virginia man, Daniel Riase, is serving a five-year prison
sentence after pleading guilty in 2007 to slipping the pills into his pregnant
girlfriend’s glass of milk.
Researchers studying the phenomenon cite several factors that lead Dominican and
other immigrant women to experiment with abortifacients: mistrust of the
health-care system, fear of surgery, worry about deportation, concern about
clinic protesters, cost and shame.
“It turns an abortion into a natural process and makes it look like a
miscarriage,” said Dr. Mark Rosing, an obstetrician at St. Barnabas Hospital in
the Bronx who led the 2000 study, which was published in the Journal of the
American Medical Women’s Association. “For people who don’t have access to
abortion for social reasons, financial reasons or immigration reasons, it
doesn’t seem like this horrible thing.”
Ms. Dominguez, for her part, said she had no insurance or money to pay for an
abortion, and could not fathom getting one for fear her mother would find out.
One of her friends had spent $1,200 on an abortion that left her with a uterine
infection, and another friend endured the procedure without anesthesia, she
said. In addition, Washington Heights is a tightknit community where abortion —
as well as birth control — is shunned; if Ms. Dominguez were spotted entering a
clinic, rumors could fly.
“There are scary moments, and you got to have a friend right next to you,” said
Ms. Dominguez, now 30 and a mother of four. “It’s cheap but dangerous. Certain
people are more delicate than others. But afterwards, I felt relief.”
A friend of Ms. Dominguez’s said her stepsister took the pills last year because
she was in the country illegally, and worried that a doctor might turn her in.
“She was just scared,” the woman said, speaking on the condition that her name
not be published to protect the stepsister’s privacy. “She had no papers, no
insurance, no nothing.”
The woman went to a free clinic afterward to make sure the pills had worked
(they had). Health care workers and other community leaders say such visits are
how they discovered widespread illicit use of the drug as well as homemade
potions.
Dr. Rosing said he learned about Cytotec during his residency at
NewYork-Presbyterian/Columbia hospital in Washington Heights, where he saw a lot
of Dominican immigrants with incomplete abortions in the emergency room. They
spoke of taking the “star pill,” a nickname for the hexagonal shape of one form
of misoprostol. He suspected “that has to be the tip of the iceberg,” he said,
“and it was.”
The pills allow pregnant women a degree of denial over what is taking place.
Like Ms. Dominguez, many women in the neighborhood talk about the need to bring
on — or “down” — their periods, not abortion. Afterward, they might tell doctors
or relatives they had lost the baby.
The Planned Parenthood study concluded that women in both nations “seemed to see
inducing the termination of pregnancy, or abortions, as a part of the reality of
their lives,” in a community where, as one interview subject put it, “we are all
doctors.” The report noted that in a culture steeped in machismo, birth control
is generally seen as the woman’s responsibility.
“If I introduce the condom into a relationship, I’m basically saying I’ve had
somebody else, and I’ve not been faithful to you,” said Haydee Morales, a vice
president at Planned Parenthood of New York.
Debralee Santos, program director at Casa Duarte, a community arts organization
in Washington Heights, said that while she had never had reason to distrust
medical professionals, she understood the apprehensions that kept other women
from seeking them out. “I get it, I really do,” she said.
“It’s a community that, even as it comes of age, always relies on itself first,”
explained Ms. Santos, who was born in the United States to immigrant parents.
“Women, in particular, continue to help each other in ways that speak to
tradition and solidarity.”
Ms. Dominguez, who volunteers at Casa Duarte and is known as Flaca, Spanish for
skinny, did not want her name or photograph published at first. But after some
thought, she decided to allow it so more people would learn about the trap many
pregnant Dominican women feel they are in.
“It’s a health risk,” she said. “There’s a lot of girls in situations like that,
and they’re overwhelmed.”
For Privacy’s Sake,
Taking Risks to End Pregnancy, NYT, 5.1.2009,
http://www.nytimes.com/2009/01/05/nyregion/05abortion.html
|